Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00085) | |||||
|---|---|---|---|---|---|
| Name |
Busulfan
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
busulfan; 55-98-1; Myleran; Busulphan; Sulphabutin; Myelosan; Leucosulfan; Busulfex; Citosulfan; Mielucin; Misulban; Mitostan; Myeloleukon; Mylecytan; Sulfabutin; Mablin; Mielevcin; Milecitan; Mielosan; Mileran; butane-1,4-diyl dimethanesulfonate; Buzulfan; 1,4-Dimesyloxybutane; 1,4-BUTANEDIOL DIMETHANESULFONATE; Myeleukon; 1,4-Butanediol, dimethanesulfonate; 1,4-Dimethanesulfonoxybutane; Tetramethylene dimethane sulfonate; Busulfano; Busulfanum; Busulphane; 1,4-Dimethylsulfonyloxybutane; 1,4-Dimethanesulfonyloxybutane; 1,4-Bis(methanesulfonoxy)butane; 1,4-Butanediol dimethylsulfonate; 1,4-Dimethylsulfonoxybutane; 1,4-Bis(methanesulfonyloxy)butane; NCI-C01592; 4-methylsulfonyloxybutyl methanesulfonate; CB 2041; AN 33501; Methanesulfonic acid, tetramethylene ester; Tetramethylene bis(methanesulfonate); 1,4-Dimethanesulphonyloxybutane; NSC 750; NSC-750; 1,4-Butanediol dimethanesulphonate; Tetramethylenester kyseliny methansulfonove; C.B. 2041; GT 2041; Tetramethylene bis[methanesulfonate]; UNII-G1LN9045DK; NSC750; 1,4-Bis[methanesulfonoxy]butane; CHEBI:28901; 2041 C. B.; G.T. 41; G1LN9045DK; 4-(methanesulfonyloxy)butyl methanesulfonate; MFCD00007562; NCGC00090905-06; Busilvex; Mitosan; DSSTox_CID_910; Busulfanum [INN-Latin]; DSSTox_RID_75857; Busulfano [INN-Spanish]; DSSTox_GSID_20910; Methanesulfonic; C6H14O6S2; CAS-55-98-1; CCRIS 418; SR-01000765405; EINECS 200-250-2; BRN 1791786; Bisulfex; AI3-25012; Busulfan;; Busulfan/Myleran; Busulfan solution; Busulfex IV; Busulfan [USP:INN:BAN:JAN]; HSDB 7605; Tetramethylenester kyseliny methansulfonove [Czech]; Prestwick_989; ACMC-20aljs; Tetramethylene {bis[methanesulfonate]}; Spectrum_000092; 2041 C.B.; Spectrum2_000067; Spectrum3_000320; Spectrum4_000259; Spectrum5_000928; CHEMBL820; NCIMech_000192; SCHEMBL4373; WLN: WS1&O4OSW1; BSPBio_001920; KBioGR_000698; KBioSS_000512; MLS001076666; Busulfan (Myleran, Busulfex); DivK1c_000847; SPECTRUM1500152; Busulfan (JP17/USP/INN); SPBio_000253; GTPL7136; DTXSID3020910; COVZYZSDYWQREU-UHFFFAOYSA-; HMS502K09; KBio1_000847; KBio2_000512; KBio2_003080; KBio2_005648; KBio3_001420; NINDS_000847; HMS1920I07; HMS2091O09; HMS2233H04; HMS3259G15; HMS3370E11; HMS3655A21; HMS3712A20; Pharmakon1600-01500152; HY-B0245; ZINC1530572; Tox21_111038; Tox21_201848; Tox21_300318; 1, {4-Bis[methanesulfonoxy]butane}; 2041CB; AC-198; BDBM50237623; CCG-35458; NSC755916; AKOS003614975; Tox21_111038_1; DB01008; KS-5212; MCULE-1840882522; NC00498; NSC-755916; IDI1_000847; NCGC00090905-01; NCGC00090905-02; NCGC00090905-03; NCGC00090905-04; NCGC00090905-05; NCGC00090905-07; NCGC00090905-08; NCGC00090905-09; NCGC00090905-10; NCGC00090905-11; NCGC00090905-12; NCGC00254038-01; NCGC00259397-01; 4-(methylsulfonyloxy)butyl methylsulfonate; NCI60_041640; SMR000058613; SBI-0051300.P003; AB0006846; FT-0623291; FT-0663910; ST45022142; SW198555-3; C06862; D00248; K-9954; 15167-EP2272827A1; 15167-EP2272832A1; 15167-EP2275420A1; 15167-EP2295055A2; 15167-EP2295416A2; 15167-EP2295426A1; 15167-EP2295427A1; 15167-EP2298748A2; 15167-EP2298764A1; 15167-EP2298765A1; 15167-EP2298778A1; 15167-EP2305642A2; 15167-EP2308833A2; 15167-EP2308861A1; 15167-EP2311453A1; 15167-EP2311825A1; 15167-EP2311842A2; 15167-EP2316832A1; 15167-EP2316833A1; 32314-EP2280012A2; 32314-EP2281815A1; 32314-EP2292595A1; 32314-EP2292615A1; 32314-EP2301928A1; 32314-EP2301933A1; 32314-EP2305640A2; 32314-EP2305671A1; 32314-EP2311827A1; 32314-EP2311840A1; 4-[(Methylsulfonyl)oxy]butyl methanesulfonate #; AB00051929-10; AB00051929-11; AB00051929_12; AB00051929_14; Busulfan, analytical standard, for drug analysis; Q348922; SR-01000765405-2; SR-01000765405-3; SR-01000765405-7; Busulfan, European Pharmacopoeia (EP) Reference Standard; Z276508890
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Chronic myelogenous leukaemia | ICD-11: 2A20 | [1] | ||
| PubChem CID | |||||
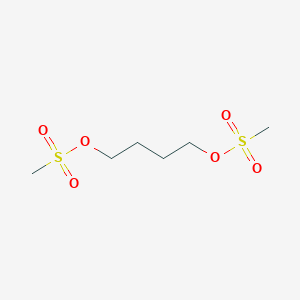
| Formula |
C6H14O6S2
|
||||
| Canonical SMILES |
CS(=O)(=O)OCCCCOS(=O)(=O)C
|
||||
| InChI |
1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3
|
||||
| InChIKey |
COVZYZSDYWQREU-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=2478"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 246.3 | Topological Polar Surface Area | 104 | |
| XlogP | -0.5 | Complexity | 294 | ||
| Heavy Atom Count | 14 | Rotatable Bond Count | 7 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 6 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Busulfan 2 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Magnesium stearate; Titanium dioxide; Triacetin; Hypromelloses; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Aspen Global | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Magnesium stearate; Titanium dioxide; Triacetin; Hypromelloses; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | GlaxoSmithKline | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
