Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00253) | |||||
|---|---|---|---|---|---|
| Name |
Ethionamide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
ethionamide; 536-33-4; 2-ethylpyridine-4-carbothioamide; Ethioniamide; Ethyonomide; Amidazine; Ethinamide; Etionamid; Etioniamid; Thioamide; Trecator; Ethylisothiamide; 2-Ethylthioisonicotinamide; Trecator-SC; 4-Pyridinecarbothioamide, 2-ethyl-; Amidazin; Ethimide; Etiocidan; Etionizin; Etionizina; Etionizine; Fatoliamid; Iridocin; Iridozin; Isotiamida; Itiocide; Nicotion; Rigenicid; Sertinon; Thianide; Thioniden; Trekator; Trescatyl; Trescazide; Tubenamide; Tubermin; Tuberoid; Tuberoson; Aetina; Aetiva; Ethina; Etimid; Etionid; Isothin; Nizotin; Teberus; Thianid; Tianid; Bayer 5312; Ethionamid prothionamid; Iridocin Bayer; Nisotin; Thiomid; 2-ETHYL-4-PYRIDINECARBOTHIOAMIDE; Etionamide [DCIT]; Ethionamidum; Etionamida; Etionamide; Tiomid; 2-Ethylisothionicotinamide; 2-Ethylisonicotinothioamide; Ethionamidum [INN-Latin]; Etionamida [INN-Spanish]; 2-Ethyl-4-thiocarbamoylpyridine; 2-Ethyl-thioisonicotinamide; 1314 TH; 2-Ethylisonicotinic acid thioamide; TH 1314; 2-ethyl-4-thiopyridylamide; NCI-C01694; UNII-OAY8ORS3CQ; 1314-Th; F.I. 58-30; 1314TH; 2-Ethyl-4-thioamidylpyridine; Isonicotinamide, 2-ethylthio-; OAY8ORS3CQ; Aethionamidum; Tio-Mid; MLS000069764; CHEBI:4885; .alpha.-Ethylisothionicotinamide; .alpha.-Ethylthioisonicotinamide; .alpha.-Ethylisonicotinoylthioamide; Trecator SC; NSC255115; NSC-255115; 1314 TN; .alpha.-Ethylisonicotinic acid thioamide; Ethina (VAN); NCGC00016497-05; CAS-536-33-4; SMR000058716; DSSTox_CID_577; DSSTox_RID_75669; DSSTox_GSID_20577; alpha-Ethylisothionicotinamide; alpha-Ethylthioisonicotinamide; alpha-Ethylisonicotinoylthioamide; 2-Ethylisonicotinic thioamide; alpha-Ethylisonicotinic acid thioamide; CCRIS 287; amino(2-ethyl(4-pyridyl))methane-1-thione; HSDB 7473; Bayer5312; SR-01000759219; EINECS 208-628-9; NSC 255115; BRN 0116474; Thiodine; Trecator (TN); Prestwick_842; Isonicotinimidic acid, 2-ethylthio-; MFCD00057361; Ethionamide [USAN:USP:INN:BAN:JAN]; PubChem15920; Spectrum_001082; CPD001370750; Opera_ID_632; Prestwick0_000526; Prestwick1_000526; Prestwick2_000526; Prestwick3_000526; Spectrum2_000994; Spectrum3_000428; Spectrum4_000547; Spectrum5_000979; 2-Ethylisonicotinothiamide; 2-Ethylisonicotinthioamide; CHEMBL1441; SCHEMBL27007; BSPBio_000511; BSPBio_002016; KBioGR_001213; KBioSS_001562; 5-22-02-00360 (Beilstein Handbook Reference); MLS001074114; MLS002454402; DivK1c_000145; SPECTRUM1500292; WLN: T6NJ B2 DYZUS; SPBio_001087; SPBio_002432; BPBio1_000563; Ethionamide (JP17/USP/INN); DTXSID0020577; AEOCXXJPGCBFJA-UHFFFAOYSA-; HMS500H07; KBio1_000145; KBio2_001562; KBio2_004130; KBio2_006698; KBio3_001236; .alpha.-Ethyl-thioisonicotinamide; Isonicotinamide, 2-ethyl, thio-; NINDS_000145; HMS1569J13; HMS1920M22; HMS2091F03; HMS2096J13; HMS2231F10; HMS2233J11; HMS3259K17; HMS3370I18; HMS3371D12; HMS3655M10; HMS3713J13; Pharmakon1600-01500292; BCP29626; HY-B0276; ZINC3872520; Tox21_110458; Tox21_202409; Tox21_302769; ANW-42028; CCG-40212; NSC757028; SBB055548; AKOS006220662; Tox21_110458_1; DB00609; FS-1770; MCULE-1322319906; NC00508; NSC-757028; IDI1_000145; NCGC00016497-01; NCGC00016497-02; NCGC00016497-03; NCGC00016497-04; NCGC00016497-06; NCGC00016497-08; NCGC00016497-09; NCGC00091074-01; NCGC00091074-02; NCGC00091074-03; NCGC00091074-04; NCGC00256600-01; NCGC00259958-01; AC-13715; AK163496; SMR001370750; SBI-0051377.P003; AB0015183; DB-049945; AB00051990; ST50949989; SW196973-3; C07665; D00591; 33010-EP2286812A1; 33010-EP2287165A2; 33010-EP2287166A2; 33010-EP2292620A2; 33010-EP2297130A1; 33010-EP2301536A1; 33010-EP2301538A1; 33010-EP2305675A1; 33010-EP2305695A2; 33010-EP2305696A2; 33010-EP2305697A2; 33010-EP2305698A2; 33010-EP2305808A1; 33010-EP2308833A2; 33010-EP2308852A1; 33010-EP2308874A1; 33010-EP2311455A1; 33010-EP2311815A1; 73833-EP2308926A1; 73833-EP2309564A1; AB00051990-09; AB00051990_10; AB00051990_11; A829694; Q414767; SR-01000759219-2; SR-01000759219-5; W-105719; BRD-K33710385-001-05-4; BRD-K51207550-001-09-9; Ethionamide, European Pharmacopoeia (EP) Reference Standard; Ethionamide, United States Pharmacopeia (USP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Human immunodeficiency virus infection | ICD-11: 1C60 | [1] | ||
| PubChem CID | |||||
| Formula |
C8H10N2S
|
||||
| Canonical SMILES |
CCC1=NC=CC(=C1)C(=S)N
|
||||
| InChI |
1S/C8H10N2S/c1-2-7-5-6(8(9)11)3-4-10-7/h3-5H,2H2,1H3,(H2,9,11)
|
||||
| InChIKey |
AEOCXXJPGCBFJA-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=2761171"></iframe>
|
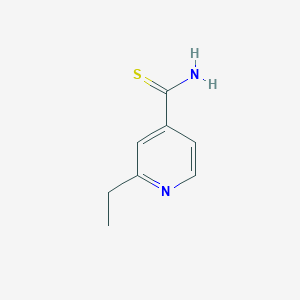 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 166.25 | Topological Polar Surface Area | 71 | |
| XlogP | 1.1 | Complexity | 147 | ||
| Heavy Atom Count | 11 | Rotatable Bond Count | 2 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Ethionamide 250 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Magnesium stearate; Talc; Titanium dioxide; Croscarmellose sodium; Silicon dioxide; Microcrystalline cellulose; Polyethylene glycol, unspecified; Polyvinyl alcohol, unspecified; Povidone, unspecified
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Pfizer | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [3] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
