Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00326) | |||||
|---|---|---|---|---|---|
| Name |
Indacaterol
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Indacaterol; 312753-06-3; QAB-149; QAB149; Arcapta; QAB 149; Onbrez; UNII-8OR09251MQ; 753498-25-8; 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one; CHEMBL1095777; CHEBI:68575; 8OR09251MQ; 312753-06-3 (free base); (R)-5-(2-(5,6-diethyl-2,3-dihydro-1H-inden-2-ylamino)-1-hydroxyethyl)-8-hydroxyquinolin-2(1H)-one; (R)-5-[2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxyquinolin-2(1H)-one; Indacaterol (USAN/INN); Indacaterol [USAN:INN:BAN]; 5-[(1R)-2-[(5,6-DIETHYL-2,3-DIHYDRO-1H-INDEN-2-YL)AMINO]-1-HYDROXYETHYL]-8-HYDROXYQUINOLIN-2(1H)-ONE; 5-{(1R)-2-((5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino)-1-hydroxyethyl}-8-hydroxyquinolin-2(1H)-one; 5-{(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl}-8-hydroxyquinolin-2(1H)-one; 1000160-87-1; QABI49; SCHEMBL48098; cc-308; GTPL7455; DTXSID90185198; HMS3886I14; BCP03766; ABP001015; BDBM50318159; MFCD18782702; ZINC35801098; AKOS024463516; BCP9000787; CCG-268560; CS-0744; DB05039; SB20619; 5-(2-(5,6-Diethylindan-2-ylamino)-1-hydroxyethyl)-8-hydroxy-1H-quinolin-2-one; NCGC00386216-07; AC-27668; AS-56318; HY-14299; BCP0726000140; D09318; AB01565805_02; 753I063; Q425654; J-521526; (R)-5-[2-(5,6-diethyl-indan-2-ylamino)-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one; 5-[(r)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1h-quinolin-2-one; 8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-ylamino)-ethyl]-1H-quinolin-2-one; 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1,2-dihydroquinolin-2-one; 5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxyquinolin-2(1H)-one; QAB 149
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Chronic obstructive pulmonary disease | ICD-11: CA22 | [1] | ||
| PubChem CID | |||||
| Formula |
C24H28N2O3
|
||||
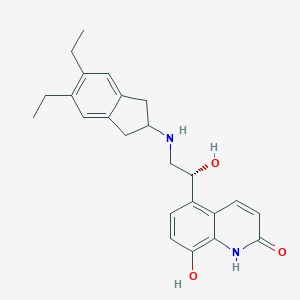
| Canonical SMILES |
CCC1=C(C=C2CC(CC2=C1)NC[C@@H](C3=C4C=CC(=O)NC4=C(C=C3)O)O)CC
|
||||
| InChI |
1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1
|
||||
| InChIKey |
QZZUEBNBZAPZLX-QFIPXVFZSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=6918554"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 392.5 | Topological Polar Surface Area | 81.6 | |
| XlogP | 3.3 | Complexity | 589 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 6 | ||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 4 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Indacaterol 0.075 mg powder | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate
|
|||||
| Dosage Form | Inhalant Powder | |||||
| Company | Novartis Pharmaceuticals Corporation; Sunovion Pharmaceuticals | |||||
| Indacaterol Maleate eq 75mcg base powder | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose Monohydrate (Contains Trace Levels Of Milk Protein)
|
|||||
| Dosage Form | Powder | |||||
| Company | Novartis | |||||
| References | |||||
|---|---|---|---|---|---|
| 1 | FDA label for approved indacaterol from the official website of the U.S. Food and Drug Administration. | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
