Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00532) | |||||
|---|---|---|---|---|---|
| Name |
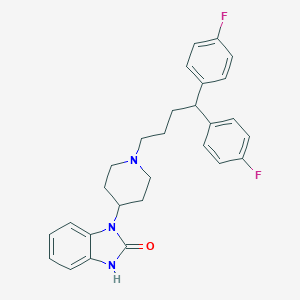
Pimozide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
pimozide; 2062-78-4; Opiran; Pimozidum; Pimozidum [INN-Latin]; McN-JR-6238; R-6238; R 6238; UNII-1HIZ4DL86F; NSC 170984; McN-JR 6238; 1-(1-(4,4-Bis(p-fluorophenyl)butyl)-4-piperidyl)-2-benzimidazolinone; 1-[1-[4,4-Bis(p-fluorophenyl)butyl]-4-piperidyl]-2-benzimidazolinone; 1HIZ4DL86F; CHEMBL1423; 1-(4,4-Bis(p-fluorophenyl)butyl)-4-(2-oxo-1-benzimidazolinyl)piperidine; 3-[1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl]-1H-benzimidazol-2-one; MLS000028410; MLS002702058; CHEBI:8212; MFCD00055081; NSC170984; NSC-170984; NCGC00015802-08; NCGC00015802-18; Primozida; SMR000058385; CAS-2062-78-4; DSSTox_CID_3474; 1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one; DSSTox_RID_77042; DSSTox_GSID_23474; Primozida [INN-Spanish]; pimozida; Primozide; C28H29F2N3O; Pimozida [INN-Spanish]; 1-[4,4-Bis(p-fluorophenyl)butyl]-4-(2-oxo-1-benzimidazolinyl)piperidine; CCRIS 9172; Orap (TN); SR-01000075392; EINECS 218-171-7; BRN 0729089; Prestwick_395; Pimozide [USAN:USP:INN:BAN:JAN]; Spectrum_000445; Tocris-0937; Opera_ID_1580; Prestwick0_000308; Prestwick1_000308; Prestwick2_000308; Prestwick3_000308; Spectrum2_001026; Spectrum3_001451; Spectrum4_000420; Spectrum5_001308; Lopac-P-1793; GTPL90; NCIMech_000356; P 1793; Lopac0_000946; SCHEMBL41584; BSPBio_000276; BSPBio_001439; BSPBio_002941; KBioGR_000720; KBioSS_000925; 5-24-02-00367 (Beilstein Handbook Reference); MLS001077311; BIDD:GT0435; DivK1c_000386; Methyl-(2-m-tolylethyl)amine; SPECTRUM1501134; Pimozide (JP17/USP/INN); SPBio_001211; SPBio_002495; BPBio1_000304; SCHEMBL7519553; DTXSID8023474; BCBcMAP01_000043; HMS501D08; KBio1_000386; KBio2_000925; KBio2_003493; KBio2_006061; KBio3_002441; AOB5780; NINDS_000386; HMS1568N18; HMS1791H21; HMS1921H19; HMS1989H21; HMS2089C11; HMS2092F09; HMS2095N18; HMS2231P23; HMS3262N14; HMS3267E19; HMS3370N13; HMS3402H21; HMS3411J16; HMS3675J16; HMS3712N18; Pharmakon1600-01501134; ZINC4175630; Tox21_110224; Tox21_301586; Tox21_500946; BDBM50334150; CCG-35918; CCG-36461; CCG-39728; NSC757854; 2-Benzimidazolinone, 1-[1-[4,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-; 3-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidyl]-1H-benzimidazol-2-one; AKOS024458706; Tox21_110224_1; DB01100; LP00946; NSC-757854; SDCCGSBI-0050920.P004; IDI1_000386; SMP1_000241; NCGC00015802-01; NCGC00015802-02; NCGC00015802-03; NCGC00015802-04; NCGC00015802-05; NCGC00015802-06; NCGC00015802-07; NCGC00015802-09; NCGC00015802-10; NCGC00015802-11; NCGC00015802-12; NCGC00015802-13; NCGC00015802-14; NCGC00015802-15; NCGC00015802-16; NCGC00015802-26; NCGC00015802-29; NCGC00016601-01; NCGC00022282-03; NCGC00024888-01; NCGC00024888-02; NCGC00024888-03; NCGC00024888-04; NCGC00024888-05; NCGC00024888-06; NCGC00024888-07; NCGC00255294-01; NCGC00261631-01; AS-13916; HY-12987; SBI-0050920.P003; AB00052215; CS-0012921; EU-0100946; FT-0673903; SW196639-3; C07566; D00560; AB00052215-06; AB00052215_07; AB00052215_08; 062P784; 117210-EP2272537A2; 117210-EP2275420A1; 117210-EP2295061A1; 117210-EP2298776A1; L000494; Q144085; WLN: T56 BMVNJ D- DT6NTJ A3YR DF&R DF; J-013477; SR-01000075392-1; SR-01000075392-3; SR-01000075392-4; SR-01000075392-6; BRD-K01292756-001-06-0; BRD-K01292756-001-13-6; Pimozide, European Pharmacopoeia (EP) Reference Standard; Z241910386; 2-Benzimidazolinone,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-; N-benzyl-N-(3-isobutoxy-2-(pyrrolidin-1-yl)propyl)benzenamine; Pimozide, United States Pharmacopeia (USP) Reference Standard; 1-[1-[4,4-Bis(p-flurophenyl)butyl]-4-piperidyl]-2-benzimidazolinone; 2H-Benzimidazol-2-one,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Schizophrenia | ICD-11: 6A20 | [1] | ||
| PubChem CID | |||||
| Formula |
C28H29F2N3O
|
||||
| Canonical SMILES |
C1CN(CCC1N2C3=CC=CC=C3NC2=O)CCCC(C4=CC=C(C=C4)F)C5=CC=C(C=C5)F
|
||||
| InChI |
1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34)
|
||||
| InChIKey |
YVUQSNJEYSNKRX-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=16362"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 461.5 | Topological Polar Surface Area | 35.6 | |
| XlogP | 6.3 | Complexity | 632 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 7 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Pimozide 1 mg tablet | Click to Show/Hide the Full List of Formulation(s): 3 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Cellulose, microcrystalline; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Teva Select Brands | |||||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Cellulose, microcrystalline; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Teva Select Brands | |||||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Cellulose, microcrystalline; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Par Pharmaceutical | |||||
| Pimozide 2 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Cellulose, microcrystalline; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Teva Select Brands | |||||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Cellulose, microcrystalline; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Avera McKennan Hospital; Par Pharmaceutical | |||||
| References | |||||
|---|---|---|---|---|---|
| 1 | FDA label for approved pimozide from the official website of the U.S. Food and Drug Administration. | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
