Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00669) | |||||
|---|---|---|---|---|---|
| Name |
Thalidomide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
thalidomide; 50-35-1; Thalomid; Sedoval; 2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione; (+/-)-THALIDOMIDE; Corronarobetin; Psycholiquid; Psychotablets; Theophilcholine; Algosediv; Asmadion; Bonbrain; Calmorex; Contergan; Distaval; Ectiluran; Enterosediv; Gastrinide; Glutanon; Hippuzon; Neosedyn; Neosydyn; Nerosedyn; Neufatin; Neurodyn; Neurosedin; Neurosedym; Nevrodyn; Noctosediv; Pantosediv; Polygripan; Profarmil; Quetimid; Quietoplex; Sandormin; Sedimide; Sedisperil; Shinnibrol; Softenil; Softenon; Talargan; Talismol; Telargean; Tensival; Thalinette; Valgraine; Asmaval; Calmore; Glupan; Grippex; Imidene; Isomin; Kevadon; Nibrol; Noxodyn; Pangul; Sleepan; Slipro; Talimol; Telagan; Thalin; Valgis; Yodomin; N-Phthaloylglutamimide; Predni-Sediv; Imida-lab; Poly-Giron; Sedalis sedi-lab; Shin-naito S; Neaufatin; Asidon 3; Pro-ban M; Imidan (peyta); N-Phthalylglutamic acid imide; Ulcerfen; 3-Phthalimidoglutarimide; alpha-Phthalimidoglutarimide; Bonbrrin; Distaxal; Distoval; Talidomida; Kedavon; (+-)-Thalidomide; 2,6-Dioxo-3-phthalimidopiperidine; Thalidomide Celgene; NSC-66847; alpha-N-Phthalylglutaramide; N-(2,6-Dioxo-3-piperidyl)phthalimide; Glutarimide, 2-phthalimido-; N-Phthalyl-glutaminsaeure-imid; alpha-(N-Phthalimido)glutarimide; 1H-Isoindole-1,3(2H)-dione, 2-(2,6-dioxo-3-piperidinyl)-; 1,3-Dioxo-2-(2,6-dioxopiperidin-3-yl)isoindoline; .alpha.-N-Phthalylglutaramide; Phthalimide, N-(2,6-dioxo-3-piperidyl)-; 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione; MFCD00153873; CHEMBL468; Thalidomide (soluble form); 2-(2,6-dioxopiperidin-3-yl)-2,3-dihydro-1H-isoindole-1,3-dione; .alpha.-Phthalimidoglutarimide; 2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione; Thalomide; (+-)-N-(2,6-Dioxo-3-piperidyl)phthalimide; CHEBI:74947; .alpha.-(N-Phthalimido)glutarimide; 2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione; NSC66847; 2-(2,6-Dioxo-piperidin-3-yl)-isoindole-1,3-dione; NSC-527179; Pro-Bam M; N-(2,6-dioxo-3-piperidinyl)phthalimide; NCGC00015989-09; Talidomide [DCIT]; Thalidomidum; Neurosedyn; Sedalis; Talidomide; Telargan; DSSTox_CID_2524; DSSTox_RID_76611; DSSTox_GSID_22524; Talidomida [INN-Spanish]; Thalidomidum [INN-Latin]; Thalidomine USP26; Synovir; Talizer; (?)-Thalidomide; Thaled; Phthalimide,6-dioxo-3-piperidyl)-; ENMD 0995; WLN: T56 BVNVJ C- DT6VMVTJ; Thalomid (TM); Thalomid (TN); 1012310-87-0; Thalidomide Pharmion; Thaled (TN); N-Phthalyl-glutaminsaeure-imid [German]; N-Phthalimidoglutamic acid imide; HSDB 3586; 1H-Isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-; SR-01000076184; EINECS 200-031-1; NSC 527179; BRN 0030233; Celgene; Pharmion; Talinol; Thalidomide (JAN/USP/INN); AI3-50606; CCRIS 8148; (y)-Thalidomide; Thalidomide,(S); CAS-50-35-1; Prestwick_463; Thalidomide [USAN:USP:INN:BAN:JAN]; (A+/-)-Thalidomide; Prestwick0_000192; Prestwick1_000192; Prestwick2_000192; Prestwick3_000192; Spectrum2_000707; Spectrum3_001715; Spectrum4_001087; Spectrum5_001791; (.+/-.)-Thalidomide; UPCMLD-DP139; Thalomid (TN) (Celgene); SCHEMBL7581; NCIOpen2_003188; Lopac0_001224; BSPBio_000143; BSPBio_001156; BSPBio_003330; KBioGR_000496; KBioGR_001474; KBioGR_002322; KBioSS_000496; KBioSS_002324; 2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione; MLS000069353; ARONIS24496; DivK1c_000051; SPECTRUM1503607; SPBio_000893; SPBio_002064; BPBio1_000159; GTPL7327; DTXSID9022524; SCHEMBL15197560; UPCMLD-DP139:001; HMS500C13; KBio1_000051; KBio2_000496; KBio2_002322; KBio2_003064; KBio2_004890; KBio2_005632; KBio2_007458; KBio3_000911; KBio3_000912; KBio3_002550; KBio3_002802; cMAP_000022; NINDS_000051; Bio1_000387; Bio1_000876; Bio1_001365; Bio2_000418; Bio2_000898; HMS1362J17; HMS1568H05; HMS1792J17; HMS1922E12; HMS1990J17; HMS2090O05; HMS2093G15; HMS2095H05; HMS2234C07; HMS3259C22; HMS3263F10; HMS3266F13; HMS3373E06; HMS3373G15; HMS3403J17; HMS3414F19; HMS3654A20; HMS3678F19; HMS3712H05; HMS3884I05; Pharmakon1600-01503607; BCP19772; NSC91729; NSC91730; Tox21_110275; Tox21_300580; Tox21_501224; (+/-)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione; 1H-Isoindole-1,3(2H)-dione, 2-(2,6-dioxo-3-piperidinyl)-, (+-)-; AC-917; ANW-53225; BBL023439; BDBM50070114; CCG-39878; NSC-91729; NSC-91730; NSC527179; NSC758479; STL356025; ( inverted question mark)-Thalidomide; AKOS009529198; Tox21_110275_1; CS-1084; DB01041; LP01224; MCULE-6327754946; NC00600; NSC-758479; SDCCGSBI-0051191.P004; (+/-)-Thalidomide, >=98%, powder; IDI1_000051; IDI1_002173; NCGC00015989-03; NCGC00015989-04; NCGC00015989-05; NCGC00015989-06; NCGC00015989-07; NCGC00015989-08; NCGC00015989-10; NCGC00015989-11; NCGC00015989-12; NCGC00015989-13; NCGC00015989-14; NCGC00015989-16; NCGC00015989-17; NCGC00015989-29; NCGC00024708-02; NCGC00024708-03; NCGC00024708-04; NCGC00024708-05; NCGC00024708-06; NCGC00024708-07; NCGC00024708-08; NCGC00024708-09; NCGC00024708-10; NCGC00024708-11; NCGC00254343-01; NCGC00261909-01; AK-91024; AS-12367; BP-30256; HY-14658; NCI60_023904; SMR000058524; SY052614; WLN: T56 BVNVJ C- DT6VMVTJ -D; WLN: T56 BVNVJ C- DT6VMVTJ -L; SBI-0051191.P003; AB0011813; DB-051759; Phthalimide,6-dioxo-3-piperidyl)-, (+)-; Phthalimide,6-dioxo-3-piperidyl)-, (-)-; AB00052362; EU-0101224; FT-0600001; FT-0602275; FT-0631211; FT-0675130; ST51039042; SW196678-4; isopropyl (3,4-dichlorophenyl)carbamodithioate; Phthalimide,6-dioxo-3-piperidyl)-, D-(+)-; Phthalimide,6-dioxo-3-piperidyl)-, L-(-)-; (+/-)-N-(2,6-dioxo-3-piperidyl)phthalimide; C07910; D00754; J90042; M-1523; AB00052362-11; AB00052362-12; AB00052362-13; AB00052362_14; AB00052362_15; 153T873; Q203174; SR-01000076184-1; SR-01000076184-3; SR-01000076184-5; SR-01000076184-8; Thalidomide N-(2,6-Dioxopiperidin-3-yl)phthalimide; W-105969; BRD-A93255169-001-04-4; BRD-A93255169-001-06-9; BRD-A93255169-001-24-2; Z1550675451; 1H-Isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-, (R)-; 1H-Isoindole-1, 2-(2,6-dioxo-3-piperidinyl)-, (S)-; 2-(2,6-dioxoazaperhydroin-3-yl)benzo[c]azoline-1,3-dione; 2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione #; (??)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione; [(R,S)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione; Thalidomide, United States Pharmacopeia (USP) Reference Standard; ( inverted question mark)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Multiple myeloma | ICD-11: 2A83 | [1] | ||
| PubChem CID | |||||
| Formula |
C13H10N2O4
|
||||
| Canonical SMILES |
C1CC(=O)NC(=O)C1N2C(=O)C3=CC=CC=C3C2=O
|
||||
| InChI |
1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17)
|
||||
| InChIKey |
UEJJHQNACJXSKW-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5426"></iframe>
|
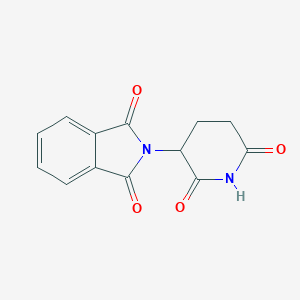 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 258.23 | Topological Polar Surface Area | 83.6 | |
| XlogP | 0.3 | Complexity | 449 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 1 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Thalidomide 100 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Celgene Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Thalidomide 150 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Celgene Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Thalidomide 200 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Celgene Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Thalidomide 50 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Celgene Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
