Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00025) | |||||
|---|---|---|---|---|---|
| Name |
Altretamine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
altretamine; 645-05-6; HEXAMETHYLMELAMINE; Hexalen; Hexastat; 2,4,6-Tris(dimethylamino)-1,3,5-triazine; Melamine, hexamethyl-; Altretaminum; Altretamina; Altretaminum [INN-Latin]; ENT 50852; NC 195; 2,4,6-Tris(dimethylamino)-s-triazine; N2,N2,N4,N4,N6,N6-hexamethyl-1,3,5-triazine-2,4,6-triamine; NCI-C50259; Altretamine (Hexalen); NSC 13875; 2-N,2-N,4-N,4-N,6-N,6-N-hexamethyl-1,3,5-triazine-2,4,6-triamine; s-Triazine, 2,4,6-tris(dimethylamino)-; UNII-Q8BIH59O7H; 1,3,5-Triazine-2,4,6-triamine, N,N,N',N',N'',N''-hexamethyl-; MLS000069621; N,N,N',N',N'',N''-hexamethyl-1,3,5-triazine-2,4,6-triamine; NSC13875; NSC-13875; Q8BIH59O7H; SMR000058181; 645-05-6 (free base); CHEBI:24564; NCGC00015100-02; DSSTox_CID_2579; DSSTox_RID_76641; DSSTox_GSID_22579; ENT-50852;RB-1515;WR-95704; Altretamina [INN-Spanish]; C9H18N6; CAS-645-05-6; Hexalen (TN); CCRIS 5492; SR-01000000117; Hexalen [Antineoplastic]; Altretamine (USP/INN); EINECS 211-428-4; BRN 0195058; Altretamine/; AI3-50852; HSDB 7559; Altretamine, solid; Altretamine [USAN:USP:INN:BAN]; KB-913; Spectrum_001308; Opera_ID_1095; Prestwick0_000946; Prestwick1_000946; Prestwick2_000946; Prestwick3_000946; Spectrum2_000907; Spectrum3_000951; Spectrum4_001064; Spectrum5_001092; Lopac-A-8723; ChemDiv2_005185; 2,4, 6-Tris(dimethylamino)-1,3,5-triazine; A 8723; N~2~,N~2~,N~4~,N~4~,N~6~,N~6~-Hexamethyl-1,3,5-triazine-2,4,6-triamine; Cambridge id 5148727; cid_2123; SCHEMBL4206; CHEMBL1455; Lopac0_000083; BSPBio_000912; KBioGR_001388; KBioSS_001788; ZINC905; MLS001076123; DivK1c_000772; SPECTRUM1503065; SPBio_000754; SPBio_003071; BPBio1_001004; GTPL7112; DTXSID4022579; BDBM37631; HMS502G14; KBio1_000772; KBio2_001788; KBio2_004356; KBio2_006924; KBio3_002042; NINDS_000772; BCPP000413; HMS1383L15; HMS1570N14; HMS1922C05; HMS2090G17; HMS2092H16; HMS2097N14; HMS2234F09; HMS3259I20; HMS3260A08; HMS3372H17; HMS3654H11; HMS3714N14; Pharmakon1600-01503065; 1,3,5-Triazine-2,4,6-triamine, N,N,N',N',N',N'-hexamethyl-; BCP27675; CCG-2238; HY-B0181; 2,6-Tris(dimethylamino)-s-triazine; Tox21_110085; Tox21_202784; Tox21_500083; ABP000596; MFCD00549245; NSC758231; STK749184; s-Triazine,4,6-tris(dimethylamino)-; AKOS001729401; Tox21_110085_1; BCP9000278; CS-2061; DB00488; KS-5247; LP00083; MCULE-1605383293; NC00595; NSC-758231; SDCCGSBI-0050071.P004; VA10242; IDI1_000772; IDI1_003900; NCGC00015100-01; NCGC00015100-03; NCGC00015100-04; NCGC00015100-05; NCGC00015100-06; NCGC00015100-07; NCGC00015100-08; NCGC00015100-09; NCGC00015100-10; NCGC00015100-11; NCGC00015100-12; NCGC00015100-14; NCGC00015100-15; NCGC00015100-23; NCGC00021216-03; NCGC00021216-04; NCGC00021216-05; NCGC00021216-06; NCGC00021216-07; NCGC00260330-01; NCGC00260768-01; AC-12006; AK431828; CAS-654-05-6; NCI60_000871; ST086739; 2,6-Tris(dimethylamino)-1,3,5-triazine; SBI-0050071.P003; AB0007992; DB-054676; AB00052308; EU-0100083; FT-0651499; SW102053-5; D02841; M-2093; 49712-EP2295426A1; 49712-EP2295427A1; 49712-EP2308833A2; 49712-EP2311825A1; AB00052308-16; AB00052308-17; AB00052308-18; AB00052308_19; AB00052308_20; 645A056; A834788; Q415242; WLN: T6N CN ENJ BN1&1 DN1&1 FN1&1; 2,4,6-Tris(dimethylamino)-1,3,5-triazine, 96%; J-523408; SR-01000000117-2; SR-01000000117-4; SR-01000000117-6; BRD-K67043667-001-04-1; BRD-K67043667-001-15-7; Z57931197; Altretamine [2,4,6-Tris(dimethylamino)-1,3,5-triazine]; [4,6-bis(dimethylamino)(1,3,5-triazin-2-yl)]dimethylamine; N2,N2,N4,N4,N6,N6-Hexamethyl-1,3,5-trazne-2,4,6-tramne; 1,5-Triazine-2,4,6-triamine, N,N,N',N',N'',N''-hexamethyl-; Altretamine, United States Pharmacopeia (USP) Reference Standard; N-[4,6-bis(dimethylamino)-1,3,5-triazin-2-yl]-N,N-dimethylamine; 1,3,5-Triazine-2,4,6-triamine, N,N,N',N',N'', N''-hexamethyl-
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Ovarian cancer | ICD-11: 2C73 | [1] | ||
| PubChem CID | |||||
| Formula |
C9H18N6
|
||||
| Canonical SMILES |
CN(C)C1=NC(=NC(=N1)N(C)C)N(C)C
|
||||
| InChI |
1S/C9H18N6/c1-13(2)7-10-8(14(3)4)12-9(11-7)15(5)6/h1-6H3
|
||||
| InChIKey |
UUVWYPNAQBNQJQ-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=2123"></iframe>
|
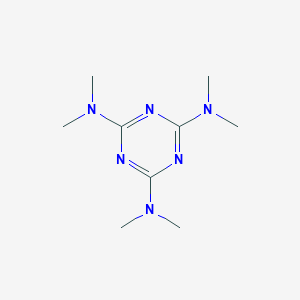 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 210.28 | Topological Polar Surface Area | 48.4 | |
| XlogP | 2.7 | Complexity | 148 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 3 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 6 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Altretamine 50 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Calcium stearate
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Eisai | |||||
| References | |||||
|---|---|---|---|---|---|
| 1 | FDA label for approved altretamine from the official website of the U.S. Food and Drug Administration. | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
