Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00402) | |||||
|---|---|---|---|---|---|
| Name |
Mesna
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
19767-45-4; Sodium 2-mercaptoethanesulfonate; Mesnex; Uromitexan; Mistabron; 2-Mercaptoethanesulfonic acid sodium salt; Mistabronco; Mucofluid; Sodium 2-mercaptoethane sulfonate; Ethanesulfonic acid, 2-mercapto-, monosodium salt; UNII-NR7O1405Q9; Mitexan; 2-mercaptoethanesulfonic acid sodium; Sodium 2-mercaptoethanesulfonate;Mesnum; sodium;2-sulfanylethanesulfonate; Mesnum; NR7O1405Q9; MFCD00007535; Ethanesulfonic acid, 2-mercapto-, sodium salt (1:1); NCGC00181166-01; DSSTox_CID_809; D 7093; D-7093; DSSTox_RID_75800; DSSTox_GSID_20809; Mesnum [INN-Latin]; C2H5NaO3S2; SMR000059219; CAS-19767-45-4; Natrium 2-mercaptoethansulfonat; CCRIS 3712; 2-Mercaptoethane sulfonate sodium; NSC-113891; EINECS 243-285-9; NSC 113891; 2-Mercaptoethanesulfonic acid sodium salt, 98%, analytical standard; 2-Mercapto-ethan-sulfonsaeure, natrium-salz; 2-Mercaptoethanesulfonic acid monosodium salt; Mesna [USAN:USP:INN:BAN]; 2-Mercaptoethanesulfonate, sodium; Uromitexan (TN); sodium 2-mercaptoethane-1-sulfonate; sodium 2-sulfanylethane-1-sulfonate; Mesnex (TN); Prestwick_1005; Spectrum2_000752; Spectrum3_001483; Spectrum4_000041; Spectrum5_001174; Mesna (JAN/USP/INN); ACMC-209f1l; CHEMBL975; SCHEMBL7993; Mesna (Uromitexan, Mesnex); KBioGR_000501; MLS001074691; MLS001333251; MLS001333252; SPECTRUM1502014; SPBio_000764; DTXSID1020809; CHEBI:31824; HMS502F17; KBio3_002565; EBD6310; HMS1921D10; HMS2092N09; HMS2236L05; HMS3259D13; HMS3372M11; HMS3655M06; HMS3715H05; sodium, 2-mercapto-ethanesulfonate; BCP14384; Tox21_112767; Tox21_200863; ANW-23767; BDBM50247978; CCG-40134; AKOS006220661; AKOS015960755; Tox21_112767_1; AC-6011; CS-1364; NC00648; NCGC00094939-01; NCGC00094939-02; NCGC00178318-04; NCGC00258417-01; 2-mercaptoethanesulphonic acid sodium salt; AK163673; AS-13260; HY-13679; AB0013231; DB-044962; SW199618-2; EN300-49858; D01459; M-2956; AB01274734-01; Q424997; Sodium 2-mercaptoethanesulfonate; Mesnaum; Mesnex; W-60283; Q-201713; 3-AMINOOXY-N-PROPYL(DIMETHYL-T-BUTYLSILYL)ETHER; Z1741968275
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Infectious cystitis | ICD-11: GC00 | [1] | ||
| PubChem CID | |||||
| Formula |
C2H5NaO3S2
|
||||
| Canonical SMILES |
C(CS(=O)(=O)[O-])S.[Na+]
|
||||
| InChI |
1S/C2H6O3S2.Na/c3-7(4,5)2-1-6;/h6H,1-2H2,(H,3,4,5);/q;+1/p-1
|
||||
| InChIKey |
XOGTZOOQQBDUSI-UHFFFAOYSA-M
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=23662354"></iframe>
|
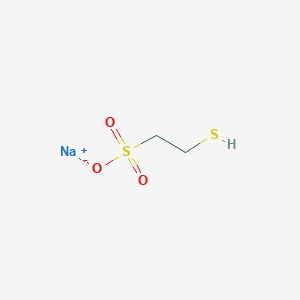 |
|||
| 3D MOL is unavailable | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 164.18 | Topological Polar Surface Area | 66.6 | |
| XlogP | N.A. | Complexity | 123 | ||
| Heavy Atom Count | 8 | Rotatable Bond Count | 2 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Mesna 400 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Magnesium stearate; Calcium phosphate, dibasic, dihydrate; Titanium dioxide; Cellulose, microcrystalline; Dimethicone; Hydroxypropyl cellulose (type h); Polyethylene glycols; Povidones; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Bayer HealthCare | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
