Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00462) | |||||
|---|---|---|---|---|---|
| Name |
Neomycin
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
neomycin; Framycetin; NEOMYCIN B; Mycifradin; Soframycin; Actiline; Fradiomycin; Neomas; Fradiomycinum; Framicetina; Framycetine; Framycetinum; Caswell No. 595; Neomycin B sulfate; Fradiomycin B; 119-04-0; NEOMYCIN SULFATE; USAF CB-19; 1404-04-2; CCRIS 5462; HSDB 3242; Soframycine; UNII-4BOC774388; Neo-Rx; Antibiotique; Enterfram; Nivemycin; Actilin; Framygen; Myacyne; Neolate; CHEBI:7508; Vonamycin powder V; Neomcin; Neomycin solution; Neomin; 4BOC774388; Neomicina [DCIT]; Framycetinum [INN-Latin]; Neomicina; Neomycin sulphate; Neomycine; Neomycinum; PIMAVECORT; Neomycine [INN-French]; Neomycinum [INN-Latin]; Framycetine [INN-French]; Framicetina [INN-Spanish]; Framycetin [INN:BAN:DCF]; Bycomycin; Jernadex; Framycetin (INN); Neomycin [INN:BAN]; EINECS 204-292-2; EINECS 215-766-3; EPA Pesticide Chemical Code 006303; BRN 0101621; Mycerin; Antibiotic 956; C23H46N6O13; NEOMYCINB; Antibiotic produced by Streptomyces decaris. Neomycin B; Prestwick3_000158; KDR Kinase Inhibitor, 3; BDBM19; SCHEMBL3279; ANTIBIOTIQUE EF 185; BSPBio_000296; GTPL709; 4-18-00-07476 (Beilstein Handbook Reference); BPBio1_000326; CHEMBL184618; DTXSID2023359; HMS2089P15; ZINC71928291; AKOS024284361; CS-6390; DB00452; NCGC00179612-01; HY-17624; ST075177; AB00443887; C01737; D05140; 30384-EP2272825A2; 30384-EP2272838A1; 30384-EP2277898A2; 30384-EP2287165A2; 30384-EP2287166A2; 30384-EP2292620A2; 30384-EP2295401A2; 30384-EP2295402A2; 30384-EP2298776A1; 30384-EP2305662A1; 30384-EP2305695A2; 30384-EP2305696A2; 30384-EP2305697A2; 30384-EP2305698A2; 30384-EP2311464A1; AB00443887-03; MYCIFRADIN; NEOMAS; PIMAVECORT; VONAMYCIN; J-004060; Q4492348; BRD-K71013094-065-01-2; UNII-I16QD7X297 component PGBHMTALBVVCIT-VCIWKGPPSA-N; 11004-65-2
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Hepatic encephalopathy | ICD-11: DB99 | [1] | ||
| PubChem CID | |||||
| Formula |
C23H46N6O13
|
||||
| Canonical SMILES |
C1[C@H]([C@@H]([C@H]([C@@H]([C@H]1N)O[C@@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CN)O)O)N)O[C@H]3[C@@H]([C@@H]([C@H](O3)CO)O[C@@H]4[C@@H]([C@H]([C@@H]([C@@H](O4)CN)O)O)N)O)O)N
|
||||
| InChI |
1S/C23H46N6O13/c24-2-7-13(32)15(34)10(28)21(37-7)40-18-6(27)1-5(26)12(31)20(18)42-23-17(36)19(9(4-30)39-23)41-22-11(29)16(35)14(33)8(3-25)38-22/h5-23,30-36H,1-4,24-29H2/t5-,6+,7-,8+,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1
|
||||
| InChIKey |
PGBHMTALBVVCIT-VCIWKGPPSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=8378"></iframe>
|
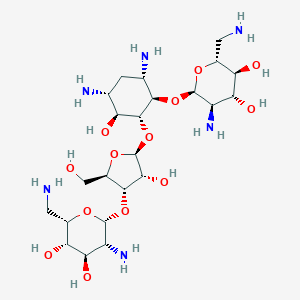 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 614.6 | Topological Polar Surface Area | 353 | |
| XlogP | -9 | Complexity | 872 | ||
| Heavy Atom Count | 42 | Rotatable Bond Count | 9 | ||
| Hydrogen Bond Donor Count | 13 | Hydrogen Bond Acceptor Count | 19 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Neomycin 500 mg tablet | Click to Show/Hide the Full List of Formulation(s): 5 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Calcium stearate; Povidone; Silicon dioxide
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | ECI Pharmaceuticals; Golden State Medical Supply | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [2] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Calcium stearate; Povidone; Alcohol
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Lannett Company | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [2] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Calcium stearate; Povidone
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Hi-Tech Pharmacal; Major Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [2] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Calcium stearate; Silicon dioxide; Povidone, unspecified
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Breckenridge Pharmaceutical | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Drug Formulation 5 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Calcium stearate; Povidone k29/32
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Teva Pharmaceuticals; X-GEN Pharmaceuticals | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
