Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00474) | |||||
|---|---|---|---|---|---|
| Name |
Nitisinone
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Nitisinone; 104206-65-7; Orfadin; 2-(2-nitro-4-(trifluoromethyl)benzoyl)cyclohexane-1,3-dione; 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione; 2-[2-nitro-4-(trifluoromethyl)benzoyl]cyclohexane-1,3-dione; SC 0735; 2-(2-Nitro-4-trifluoromethylbenzoyl)cyclohexane-1,3-dione; Nitisinone-13C6; Nitisone; UNII-K5BN214699; 2-(Alpha,alpha,alpha-trifluoro-2-nitro-p-tuluoyl)-1,3-cyclohexanedione; MFCD01752192; SC-0735; CHEMBL1337; CHEBI:50378; 1,3-Cyclohexanedione, 2-(2-nitro-4-(trifluoromethyl)benzoyl)-; 1,3-Cyclohexanedione, 2-[2-nitro-4-(trifluoromethyl)benzoyl]-; K5BN214699; 2-{[2-nitro-4-(trifluoromethyl)phenyl]carbonyl}cyclohexane-1,3-dione; Nitisinone [USAN:INN]; nitisinona; nitisinonum; 1246815-63-3; SMR002529592; Orfadin (TN); C14H10F3NO5; Nitisinone (JAN/USAN/INN); PubChem7541; FE-0200; BIDD:PXR0129; cc-114; MLS004774025; MLS006011955; SCHEMBL338795; AMBZ0071; GTPL6834; DTXSID9042673; Nitisinone, >=95% (HPLC); SCHEMBL15996621; 2-[2-Nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione; HMS3740A15; HMS3870K03; BCP15276; HY-B0607; ABP000997; ANW-54114; BDBM50088804; NSC773149; QC-508; RB3134; AKOS015891363; AKOS015994590; ZINC100014475; ACN-035825; AM62666; CCG-222085; DB00348; EX-6233; MCULE-6109734568; MP-1670; NSC-773149; SB19017; NCGC00185778-01; NCGC00185778-02; NCGC00185778-04; NCGC00185778-07; AC-26934; AK-55736; SY047291; AB0034533; DB-014936; FT-0672739; D05177; A800922; SR-01000940576; J-505680; Q3877355; SR-01000940576-2; NTBC; Nitisone; SC0735; SC 0735; SC-0735; Z1514110653; 1,3-Cyclohexanedione,2-[2-nitro-4-(trifluoromethyl)benzoyl]-; 2-(2-Nitro-4-(trifluoromethyl)-benzoyl)cyclohexane-1,3-dione; 2-[2-nitro-4-(trifluoromethyl)phenyl]carbonylcyclohexane-1,3-dione; 2-[[2-nitro-4-(trifluoromethyl)phenyl]-oxomethyl]cyclohexane-1,3-dione
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Phenylketonuria | ICD-11: 5C50 | [1] | ||
| PubChem CID | |||||
| Formula |
C14H10F3NO5
|
||||
| Canonical SMILES |
C1CC(=O)C(C(=O)C1)C(=O)C2=C(C=C(C=C2)C(F)(F)F)[N+](=O)[O-]
|
||||
| InChI |
1S/C14H10F3NO5/c15-14(16,17)7-4-5-8(9(6-7)18(22)23)13(21)12-10(19)2-1-3-11(12)20/h4-6,12H,1-3H2
|
||||
| InChIKey |
OUBCNLGXQFSTLU-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=115355"></iframe>
|
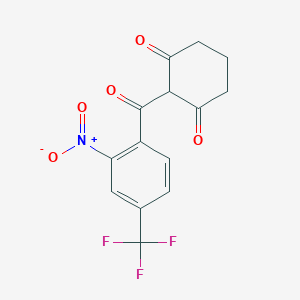 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 329.23 | Topological Polar Surface Area | 97 | |
| XlogP | 2.3 | Complexity | 524 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 2 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 8 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Nitisinone 10 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Glyceryl dibehenate
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Cycle Pharmaceuticals | |||||
| Nitisinone 2 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Glyceryl dibehenate
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Cycle Pharmaceuticals | |||||
| Nitisinone 5 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Glyceryl dibehenate
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Cycle Pharmaceuticals | |||||
| Nitisinone 10 mg capsule | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Rare Disease Therapeutics | |||||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Swedish Orphan Biovitrum AB | |||||
| Nitisinone 2 mg capsule | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Rare Disease Therapeutics | |||||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Swedish Orphan Biovitrum AB | |||||
| Nitisinone 20 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Swedish Orphan Biovitrum AB | |||||
| Nitisinone 5 mg capsule | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Rare Disease Therapeutics | |||||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Titanium dioxide; Gelatin; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Swedish Orphan Biovitrum AB | |||||
| References | |||||
|---|---|---|---|---|---|
| 1 | FDA label for approved nitisinone from the official website of the U.S. Food and Drug Administration. | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
