Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00545) | |||||
|---|---|---|---|---|---|
| Name |
Potassium iodide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
potassium iodide; 7681-11-0; Potassium iodide (KI); Kali iodide; Thyro-Block; Potassium monoiodide; Knollide; Asmofug E; Potassium diiodide; Dipotassium diiodide; Kaliumiodid; potassium;iodide; Potassium iodide (K2I2); Potassium iodide (K(I2)); potassiumiodide; UNII-1C4QK22F9J; MFCD00011405; 1C4QK22F9J; CHEBI:8346; NSC-77362; Thyroshield; Joptone; Potide; Kalii iodidum; Potassium iodide solution; Iodure de potassium; Tripotassium triiodide; Caswell No. 694; iodopotassium; Thyrosafe; Kalium iodatum; potassium-iodide; Iodine Solution, 0.1N (N/10); Potassium iodide (K3I3); Potassium iodide, 99+%, ACS reagent; Potassium iodide, 99+%, for analysis; Potassium iodide, 99%, for biochemistry; HSDB 5040; Potassium iodide, 99%, extra pure, briquettes; EINECS 231-659-4; NSC 77362; EPA Pesticide Chemical Code 075701; Kaliumjodid; potasium iodide; potassium iodid; Potassium iodide [USP:JAN]; Potassium iodide, 99.995%, (trace metal basis), extra pure; AI3-52931; CCRIS 8168; Potassium salt of hydriodic acid; Thyroblock (TN); potassium iodide ts; Potassium Iodide AR; Gold etchant, standard; Potassium Iodide,(S); ACMC-1BDBB; Nesslers Reagent Solution; Potassium Iodide USP/FCC; Potassium iodide ACS grade; WLN: KA I; EC 231-659-4; Potassium iodide, ultra dry; CHEMBL1141; DSSTox_CID_14836; DSSTox_RID_79208; DSSTox_GSID_34836; Potassium Iodide Neutral ACS; Potassium iodide, ACS reagent; Potassium iodide (JP17/USP); DTXSID7034836; Potassium iodide, LR, >=99%; HMS3651G04; CS-B1801; K I (H2 O)2; NSC77362; Potassium iodide, AR, >=99.8%; Tox21_301293; ANW-46028; Mercuric and potassium iodide solution; AKOS015833375; CCG-266323; DB06715; LS41972; Potassium iodide, BioXtra, >=99.0%; NCGC00257542-01; Potassium iodide solution, 15 % (w/v); Potassium iodide, ReagentPlus(R), 99%; CAS-7681-11-0; Potassium iodide, Vetec(TM) reagent grade; Potassium iodide, ACS reagent, >=99.0%; Potassium iodide, plant cell culture tested; FT-0645116; Potassium iodide, 99.999% (metals basis); SW219147-1; Potassium iodide, BioUltra, >=99.5% (AT); Potassium iodide, tested according to Ph.Eur.; Potassium iodide, Trace metals grade 99.99%; C08219; D01016; Potassium iodide, SAJ first grade, >=99.5%; Y-9233; AB01274864-01; AB01568251_01; Potassium iodide, >=99.99% trace metals basis; Potassium iodide, JIS special grade, >=99.5%; Potassium iodide, purum p.a., >=99.0% (AT); Q121874; Potassium iodide, puriss. p.a., ACS reagent, >=99.0% (AT); Potassium iodide, suitable for oxidant determination, >=99.5%; Potassium iodide, anhydrous, beads, -10 mesh, 99.998% trace metals basis; Potassium iodide, puriss. p.a., reag. ISO, reag. Ph. Eur., >=99.5%; Potassium iodide, United States Pharmacopeia (USP) Reference Standard; Potassium iodide, anhydrous, free-flowing, Redi-Dri(TM), ACS reagent, >=99%; Potassium iodide, anhydrous, free-flowing, Redi-Dri(TM), ReagentPlus(R), 99%
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Thyrotoxicosis | ICD-11: 5A02 | [1] | ||
| PubChem CID | |||||
| Formula |
IK
|
||||
| Canonical SMILES |
[K+].[I-]
|
||||
| InChI |
1S/HI.K/h1H;/q;+1/p-1
|
||||
| InChIKey |
NLKNQRATVPKPDG-UHFFFAOYSA-M
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4875"></iframe>
|
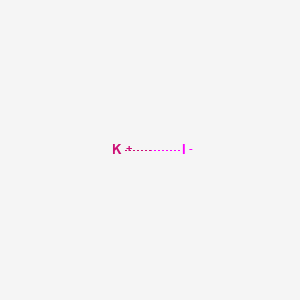 |
|||
| 3D MOL is unavailable | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 166.003 | Topological Polar Surface Area | 0 | |
| XlogP | N.A. | Complexity | 2 | ||
| Heavy Atom Count | 2 | Rotatable Bond Count | 0 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 1 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Potassium iodide 130 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Sodium thiosulfate anhydrous; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Anbex | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Potassium iodide 65 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Sodium thiosulfate anhydrous; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Anbex | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Magnesium stearate; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Recipharm AB | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
