Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00596) | |||||
|---|---|---|---|---|---|
| Name |
Rimantadine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
rimantadine; 13392-28-4; 1-(1-Adamantyl)ethanamine; 1-(Adamantan-1-yl)ethanamine; 1-Rimantadine; 1-(adamantan-1-yl)ethan-1-amine; alpha-Methyl-1-adamantanemethylamine; alpha-Methyladamantanemethylamine; RIMANTADIN; 1-Adamantan-1-yl-ethylamine; CHEMBL959; 1-Adamantanemethylamine, .alpha.-methyl-; .alpha.-Methyladamantanemethylamine; 1-(1-adamantyl)-ethylamine; 1-(tricyclo[3.3.1.1~3,7~]dec-1-yl)ethanamine; Rimantadina; Rimantadinum; [1-(1-adamantyl)ethyl]amine hydrochloride; Rimantadine [INN:BAN]; Rimantadinum [INN-Latin]; 1-(1-Adamantyl)ethylamin; Rimantadina [INN-Spanish]; Tricyclo[3.3.1.13,7]decane-1-methanamine, a-methyl-; (R)-1-(1-Adamantyl)ethylamine; Rimant; 1-(1-adamantyl)ethylamine; (1S)-1-(adamantan-1-yl)ethan-1-amine; Rimantadine (INN); HSDB 7438; Enamine_005755; NCGC00159491-02; Rimant & .alpha. IFN; Rimantadine (Flumadine); Rimantidine & .alpha.IFN; 1-Adamantan-1-ylethylamine; BRN 2715740; rimantidin; Rimantadin A; 887336-05-2; 1-adamantanylethylamine; Tricyclo(3.3.1.13,7)decane-1-methanamine, alpha-methyl-; Maybridge1_002066; Tricyclo(3.3.1.1(sup 3,7))decane-1-methanamine, alpha-methyl-; 1-ADAMANTANEMETHYLAMINE, alpha-METHYL-; SCHEMBL2981; 1-tricyclo[3.3.1.1~3,7~]dec-1-ylethanamine; Oprea1_602732; SCHEMBL2619249; CHEMBL1201272; DTXSID2023561; SCHEMBL20409367; CHEBI:94440; HMS1410F13; HMS2090L19; HMS3604N13; HMS3655J05; ALBB-013870; BCP12269; HY-B0338; ANW-72018; BBL013215; BDBM50216627; STK177253; (alpha-methyl-1-adamantyl)methylamine; AKOS000264537; AKOS006238592; AKOS016038537; .alpha.-Methyl-1-adamantanemethylamine; AM84461; CCG-236078; DB00478; MCULE-9027470290; 4-Bromo-7-(trifluoromethyl)- quinoline; IDI1_007990; NCGC00159491-03; NCGC00159491-05; AS-68744; SBI-0206810.P001; AB0012750; DB-042207; FT-0630403; ST45025920; SW220023-1; C07236; D08483; Q42171; 1-[(3R,5S,7s)-adamantan-1-yl]ethan-1-amine; AB00638368-09; AB00959689-03; AB01506092_02; AB01506092_03; 392R284; BRD-A84282119-003-01-2; Z56757137; Tricyclo(3.3.1.1^3,7)decane-1-methanamine, .alpha.-methyl-; Tricyclo(3.3.1.1(sup 3,7))decane-1-methanamine, .alpha.-methyl-; Tricyclo[3,3,1,1(3,7)]decane-1-methanamine, .alpha.-methyl-; Tricyclo(3.3.1.1^3,7)decane-1-methanamine, .alpha.-methyl- & IFN.alpha
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Influenza virus infection | ICD-11: 1E30 | [1] | ||
| PubChem CID | |||||
| Formula |
C12H21N
|
||||
| Canonical SMILES |
CC(C12CC3CC(C1)CC(C3)C2)N
|
||||
| InChI |
1S/C12H21N/c1-8(13)12-5-9-2-10(6-12)4-11(3-9)7-12/h8-11H,2-7,13H2,1H3
|
||||
| InChIKey |
UBCHPRBFMUDMNC-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5071"></iframe>
|
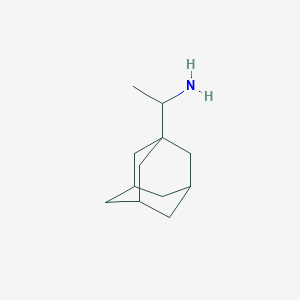 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 179.3 | Topological Polar Surface Area | 26 | |
| XlogP | 2.6 | Complexity | 180 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 1 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 1 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Rimantadine 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Magnesium stearate; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycol; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Caraco Pharma; H. J. Harkins Company | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Magnesium stearate; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycols; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Bryant Ranch Prepack; Carilion Materials Management; Impax Generics; Stat Rx USA | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Rimantadine Hydrochloride 100mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Hypromelloses; Magnesium Stearate; Cellulose; Microcrystalline; Sodium Starch Glycolate Type A Potato; Fd&C Yellow No. 6; Polyethylene Glycol
|
|||||
| Dosage Form | Tablet | |||||
| Company | Amneal Pharmaceuticals Of New York | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Hypromellose | DIG Info | Cytochrome P450 3A5 (IC50 = 19.4 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Polyethylene glycol 4000 | DIG Info | Albendazole monooxygenase (Inhibition ratio < 40 %) | [5] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
