Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00642) | |||||
|---|---|---|---|---|---|
| Name |
Sulfadiazine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
sulfadiazine; 68-35-9; Sulphadiazine; Sulfapyrimidine; Sulfadiazin; Adiazine; Sulfazine; Sulfadiazene; Adiazin; Debenal; Liquadiazine; Sulfapyrimidin; Pyrimal; 2-Sulfanilamidopyrimidine; Cremodiazine; Spofadrizine; Theradiazine; Cremotres; Deltazina; Diazolone; Eskadiazine; Microsulfon; Neotrizine; Palatrize; Piridisir; Quadetts; Quadramoid; Sanodiazine; Sterazine; Sulfatryl; Sulfolex; Sulfonsol; Terfonyl; Trifonamide; Truozine; Diazin; Neazine; Pirimal; Sulfose; Trisem; Sulfanilamidopyrimidine; Honey diazine; Lipo-Levazine; Tri-Sulfameth; Triple Sulfas; Coco-Diazine; Lipo-Diazine; Metha-Meridiazine; Sulfadiazinum; Diazovit; Sulfadiazina; Sulphadiazine E; Sulfapirimidin; Di-Azo-Mul; Thi-Di-Mer; 2-Sulfanilylaminopyrimidine; Codiazine; Silvadene; Pecta-diazine, suspension; 4-amino-N-(pyrimidin-2-yl)benzenesulfonamide; Benzenesulfonamide, 4-amino-N-2-pyrimidinyl-; 4-amino-N-pyrimidin-2-ylbenzenesulfonamide; RP 2616; Pyrimidine, 2-sulfanilamido-; 2-Sulfapyrimidine; N(1)-2-pyrimidylsulfanilamide; N(1)-2-pyrimidinylsulfanilamide; 4-AMINO-N-2-PYRIMIDINYLBENZENESULFONAMIDE; 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide; MFCD00006065; Thermazene; CHEBI:9328; Sulfanilamide, N(sup 1)-2-pyrimidinyl-; N(sup1)-2-Pyrimidylsulfanilamide; Sulfanilamide, N1-2(1H)-pyrimidinylidene-; 4-amino-N-pyrimidin-2-yl-benzenesulfonamide; Sulfanilamide, N1-2-pyrimidinyl-; UNII-0N7609K889; N(sup1)-2-Pyrimidinylsulfanilamide; 4-amino-N-(pyrimidin-2-yl)benzene-1-sulfonamide; CHEMBL439; S. N. 112; N(1)-(2-Pyrimidinyl)sulfanilamide; NSC35600; NSC-35600; CAS-68-35-9; NCGC00016305-01; Solfadiazina; CocoDiazine; Solfadiazina [DCIT]; 0N7609K889; [(4-aminophenyl)sulfonyl]pyrimidin-2-ylamine; 2-Sulfanilamidopyrimidin; NSC117870; Sulfapyrimidin [German]; Sulfadiazinum [INN-Latin]; Sulfadiazina [INN-Spanish]; Sildaflo; S.N. 112; 2-Sulfanilamidopyrimidin [German]; A-306 (VAN); N(sup 1)-2-Pyrimidinylsulfanilamide; 141582-64-1; SMR000059113; Sulfadiazine (TN); N-(2-Pyrimidinyl)sulfanilamide; rBPI21 & Sulfa; 4-Amino-N-2-pyrimidinyl-benzenesulfonamide; RP-2616; N1-(Pyrimidin-2-yl)sulfanilamide; SR-01000002973; EINECS 200-685-8; NSC 35600; 2-Sulfanilamido-pyrimidine; BRN 0235192; diazine; CRL-8131 & Sulfadiazine; N1-2-Pyrimidinylsulfanilamide; Sulfadiazine (JAN/USP/INN); AI3-01047; Sulfadiazine,(S); Trisulfapyrimidine, oral suspension; Sulfadiazine [USP:INN:BAN:JAN]; Prestwick_428; Spectrum_000986; Sulfadiazina Reig Jofre; Sulfacombin (Salt/Mix); Prestwick0_000023; Prestwick1_000023; Prestwick2_000023; Prestwick3_000023; Spectrum2_001319; Spectrum3_001362; Spectrum4_000342; Spectrum5_000992; Epitope ID:140083; N1-2-Pyrimidylsulfanilamide; Sulfadiazine, >=99.0%; DSSTox_CID_24130; DSSTox_RID_80105; DSSTox_GSID_44130; Oprea1_081078; SCHEMBL24176; BSPBio_000085; BSPBio_002884; KBioGR_000743; KBioSS_001466; 5-25-10-00067 (Beilstein Handbook Reference); MLS000069423; MLS006011457; DivK1c_000543; SPECTRUM1500546; SPBio_001417; SPBio_002006; BPBio1_000095; WLN: T6N CNJ BMSWR DZ; DTXSID7044130; HMS501L05; KBio1_000543; KBio2_001466; KBio2_004034; KBio2_006602; KBio3_002104; NINDS_000543; HMS1568E07; HMS1921A13; HMS2090P09; HMS2092I15; HMS2095E07; HMS2235D19; HMS3371L19; HMS3655I10; HMS3712E07; Pharmakon1600-01500546; ZINC120319; ALBB-014888; BCP12140; HY-B0273; Tox21_110360; BBL013169; BDBM50166571; CCG-39257; NSC757324; Recombinant bactericidal/permeability-increasing protein & Sulfadiazine; SBB007604; SBB057674; STK317797; 2-(p-Aminobenzenesulfonamido)pyrimidin; AKOS000119073; DB00359; KS-1144; MCULE-4577338719; NE10425; NSC-757324; 2-(4-Aminobenzenesulfonamido)pyrimidine; 4-amino-N-2-pyrimidylbenzenesulfonamide; IDI1_000543; NCGC00016305-02; NCGC00016305-03; NCGC00016305-04; NCGC00016305-05; NCGC00016305-06; NCGC00016305-09; NCGC00016305-10; NCGC00016305-11; NCGC00023291-03; NCGC00023291-04; SULFADIAZINE (TRISULFAPYRIMIDINES); 2-(4-Aminobenzenesulfonylamino)pyrimidine; AC-26817; AK321973; ST059447; Sulfanilamide, N1-2-pyrimidinyl- (8CI); 4-amino-N-2-pyrimidinyl benzenesulfonamide; Mixture of sulfadiazine, and sulfamethazine; SBI-0051520.P003; Sulfadiazine 100 microg/mL in Acetonitrile; 4-[[(Pyrimidin-2-yl)amino]sulfonyl]aniline; AB00052095; Benzenesulfonamide,4-amino-N-2-pyrimidinyl-; FT-0674739; FT-0674740; FT-0674741; ST51006797; SW196657-3; 4-amino-N-(2-pyrimidinyl) benzenesulfonamide; 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide #; 68S359; C07658; D00587; Trisulfapyrimidine, oral suspension (Salt/Mix); AB00052095-13; AB00052095-14; AB00052095_15; AB00052095_16; Benzenesulfonamide, 4-amino-N-(2-pyrimidinyl)-; Sulfadiazine, VETRANAL(TM), analytical standard; Q-201759; Q2555060; SR-01000002973-2; SR-01000002973-3; BRD-K32273377-001-05-4; BRD-K32273377-001-09-6; F1657-1720; Sulfadiazine, certified reference material, TraceCERT(R); Z271004844; Sulfadiazine, European Pharmacopoeia (EP) Reference Standard; Sulfadiazine, United States Pharmacopeia (USP) Reference Standard; 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, N1-(Pyrimidin-2-yl)sulfanilamide; Sulfadiazine, Pharmaceutical Secondary Standard; Certified Reference Material
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Toxoplasmosis | ICD-11: 1F57 | [1] | ||
| PubChem CID | |||||
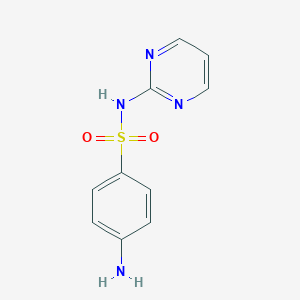
| Formula |
C10H10N4O2S
|
||||
| Canonical SMILES |
C1=CN=C(N=C1)NS(=O)(=O)C2=CC=C(C=C2)N
|
||||
| InChI |
1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14)
|
||||
| InChIKey |
SEEPANYCNGTZFQ-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5215"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 250.28 | Topological Polar Surface Area | 106 | |
| XlogP | -0.1 | Complexity | 327 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 3 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Sulfadiazine 500 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Sodium benzoate; Docusate sodium; Croscarmellose sodium; Cellulose, microcrystalline; Povidone k12; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | RemedyRepack | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [2] | |||
| Sodium benzoate | DIG Info | Carbonic anhydrase II (Ki = 30000 nM) | [3] | |||
| Docusate sodium | DIG Info | Solute carrier SLCO2B1 (Ki = 2.3 uM) | [2] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Sodium benzoate; Docusate sodium; Croscarmellose sodium; Cellulose, microcrystalline; Povidones; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Eon Labs | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [2] | |||
| Sodium benzoate | DIG Info | Carbonic anhydrase II (Ki = 30000 nM) | [3] | |||
| Docusate sodium | DIG Info | Solute carrier SLCO2B1 (Ki = 2.3 uM) | [2] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
