Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00655) | |||||
|---|---|---|---|---|---|
| Name |
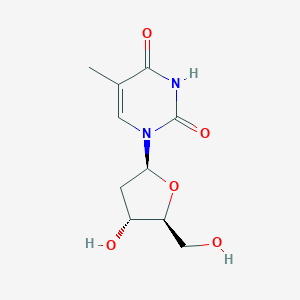
Telbivudine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Telbivudine; Epavudine; 2'-Deoxy-L-thymidine; L-Thymidine; beta-L-Thymidine; 3424-98-4; Telbivudin; Tyzeka; L-Deoxythymidine; Sebivo; NV-02B; LDT600; beta-L-2'-Deoxythymidine; NV 02B; UNII-2OC4HKD3SF; CHEBI:63624; LDT-600; 1-(2-Deoxy-beta-L-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione; 2OC4HKD3SF; 26879-47-0; 1-((2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; 1-(2-deoxy-beta-L-ribofuranosyl)-5-methyluracil; MFCD02683612; 1-[(2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione; Tyzeka (TN); Telbivudine (USAN/INN); Telbivudine [USAN:INN:BAN]; 5-methyl-l-uridine; 1-[(2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione; b-L-2'-deoxy-thymidine; SCHEMBL124279; Telbivudine (Sebivo, Tyzeka); CHEMBL374731; Epavudine;L-Thymidine;NV 02B; Thymine, 1-(2-deoxy-beta-L-erythro-pentofuranosyl)-; ZINC2159; Telbivudine, >=98% (HPLC); DTXSID30187813; HY-B0017; BDBM50088372; AKOS025117349; AC-5632; CCG-266887; CS-1934; DB01265; SB20806; 1-(2-Deoxy-beta-L-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-Pyrimidinedione; 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-b-L-erythro-pentofuranosyl)-5-methyl-; NCGC00346560-01; NCGC00346560-06; 1-[(2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione; AS-35209; SW220236-1; D06675; AB01274717-01; AB01274717_02; Q413621; BRD-K15976406-001-01-7; Thymine, 1-(2-deoxy-.beta.-L-erythro-pentofuranosyl)-; Z2574360267; 1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione; 1-((2S,4S,5S)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; L-thymidine; beta-L-thymidine; L-dT; 2'-deoxy-L-thymidine; 1-(2-deoxy-beta-L-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Viral hepatitis | ICD-11: 1E51 | [1] | ||
| PubChem CID | |||||
| Formula |
C10H14N2O5
|
||||
| Canonical SMILES |
CC1=CN(C(=O)NC1=O)[C@@H]2C[C@H]([C@@H](O2)CO)O
|
||||
| InChI |
1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16)/t6-,7+,8+/m1/s1
|
||||
| InChIKey |
IQFYYKKMVGJFEH-CSMHCCOUSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=159269"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 242.23 | Topological Polar Surface Area | 99.1 | |
| XlogP | -1.2 | Complexity | 381 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 2 | ||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Telbivudine 600 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Talc; Titanium dioxide; Silicon dioxide; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycols; Povidones; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Novartis Pharmaceuticals Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
