Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00732) | |||||
|---|---|---|---|---|---|
| Name |
Vorapaxar
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Vorapaxar; 618385-01-6; Zontivity; Sch 530348; SCH-530348; SCH530348; Vorapaxar free base; UNII-ZCE93644N2; MK-5348; CHEMBL493982; CHEBI:82702; ZCE93644N2; 705260-08-8 (sulfate); 618385-01-6 (free base); [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-carbamic acid ethyl ester; ethyl n-[(3r,3as,4s,4ar,7r,8ar,9ar)-4-[(e)-2-[5-(3-fluorophenyl)-2-pyridyl]vinyl]-3-methyl-1-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3h-benzo[f]isobenzofuran-7-yl]carbamate; Ethyl [(1r,3ar,4ar,6r,8ar,9s,9as)-9-{(E)-2-[5-(3-Fluorophenyl)pyridin-2-Yl]ethenyl}-1-Methyl-3-Oxododecahydronaphtho[2,3-C]furan-6-Yl]carbamate; Ethyl N-[(3R,3aS,4S,4aR,7R,8aR,9aR)-4-[(E)-2-[5-(3-fluorophenyl)-2-pyridyl]vinyl]-3-methyl-1-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-7-yl]carbamate sulfate; Vorapaxar [USAN:INN]; an-6-yl]carbamate; Carbamic acid, ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((1E)-2-(5-(3-fluorophenyl)-2- pyridinyl)ethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-c)furan-6-yl)-, ethyl ester; Carbamic acid, [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)-2- pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-, ethyl ester; ethyl N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl]-1-methyl-3-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-1H-benzo[f][2]benzofuran-6-yl]carbamate; Vorapaxar (USAN/INN); MLS006010324; SCHEMBL471187; GTPL4047; SCHEMBL3399110; Vorapaxar (SCH 530348); C29H33FN2O4; DTXSID201009336; EX-A1343; ZINC3925861; BDBM50261110; MFCD16038876; ACN-048227; CCG-269633; CS-5527; DB09030; MK 5348; AS-56098; ethyl ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(5-(3-fluorophenyl)pyridin-2-yl)vinyl)-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl)carbamate; HY-10119; SMR004701388; D09765; A1-03410; Q7941753; (3R,3aalpha,4abeta,8aalpha,9aalpha)-3alpha-Methyl-4beta-[(E)-2-[5-(3-fluorophenyl)-2-pyridyl]ethenyl]-7beta-(ethoxycarbonylamino)dodecahydronaphtho[2,3-c]furan-1-one; [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-{(E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl}-1-methyl-3-oxododecahydronaphtho[2,3-c]fur; Carbamic acid, N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E)-2-[5-(3-fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-, ethyl ester; Ethyl ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((1E)-2-(5-(3-fluorophenyl)pyridin-2-yl)ethenyl)- 1-methyl-3-oxododecahydronaphtho(2,3-c)furan-6-yl)carbamate; ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(5-(3-fluorophenyl)pyridin-2-yl)vinyl)-1-methyl-3-oxododecahydronaphtho[2,3-C]furan-6-ylcarbamate; Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-2-[5-(3-fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1(R)-methyl-3-oxonaphtho[2,3-c]furan-6(R)-yl]carbamate; Ethyl N-((1R,3aR,4aR,6R,8aR,9S)-9-((E)-2-(5-(3-fluorophenyl)pyridin-2-yl)ethenyl)-1-methyl-3-oxo-decahydro-1H-naphtho(2,3-c)furan-6-yl)carbamate; ethyl N-[(3R,3aS,4S,4aR,7R,8aR,9aR)-4-[(E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl]-3-methyl-1-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-naphtho[6,7-c]furan-7-yl]carbamate
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Myocardial infarction | ICD-11: BA41 | [1] | ||
| PubChem CID | |||||
| Formula |
C29H33FN2O4
|
||||
| Canonical SMILES |
CCOC(=O)N[C@@H]1CC[C@@H]2[C@@H](C1)C[C@@H]3[C@H]([C@H]2/C=C/C4=NC=C(C=C4)C5=CC(=CC=C5)F)[C@H](OC3=O)C
|
||||
| InChI |
1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/b12-9+/t17-,20+,23-,24-,25+,26-,27+/m1/s1
|
||||
| InChIKey |
ZBGXUVOIWDMMJE-QHNZEKIYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=10077130"></iframe>
|
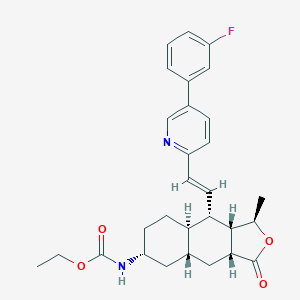 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 492.6 | Topological Polar Surface Area | 77.5 | |
| XlogP | 5.3 | Complexity | 821 | ||
| Heavy Atom Count | 36 | Rotatable Bond Count | 6 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Vorapaxar Sulfate eq 2.08mg base tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose Monohydrate; Microcrystalline Cellulose; Croscarmellose Sodium; Povidone; Magnesium Stearate; Lactose Monohydrate; Hypromellose; Titanium Dioxide; Triacetin (Glycerol Triacetate); Iron Oxide Yellow
|
|||||
| Dosage Form | Tablet | |||||
| Company | Xspire Pharma | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Hypromellose | DIG Info | Cytochrome P450 3A5 (IC50 = 19.4 uM) | [2] | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [3] | |||
| Vorapaxar 2.08 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Magnesium stearate; Ferric oxide yellow; Titanium dioxide; Triacetin; Croscarmellose sodium; Cellulose, microcrystalline; Hypromellose 2910 (15 mpa.s); Povidones
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Aralez Pharmaceuticals; Merck Sharp & Dohme | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [3] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
