| Synonyms |
Click to Show/Hide the Synonyms of This DIG
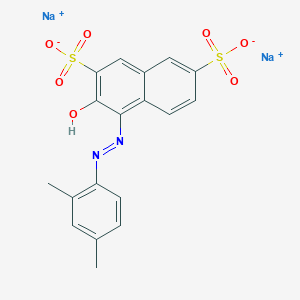
Acid Red 26; 3761-53-3; PONCEAU MX; Xylidine ponceau 2R; Xylidine Ponceau; Ponceau R; Ponceau xylidine; Ponceau 2R; Ponceau de Xylidine; C.I. Acid Red 26; Xylidine Red; CI F Food Red 5; Ponceau Red R; Ponceau G; Brilliant ponceau G; Lake ponceau; Ponceau fr; Ponceau gr; Ponceau nr; Ponceau rg; Acid Scarlet; Ponceau Red; Calcocid 2ril; C.I. Food Red 5; Ponceau J; Ponceaux MX; Kiton Ponceau R; Ponceau RR; Ponceau RS; Scarlet 2rl; Acetacid Red J; Acid Ponceau R; Java ponceau 2r; Lake Scarlet R; Ponceau BNA; Ponceau PXM; Scarlet RRA; Acidal Ponceau G; Edicol ponceau rs; Neklacid Red RR; Ponceau 2Re; Acid Scarlet 2B; Acid Scarlet 2R; Aizen Ponceau RH; Scarlet 2R; Acilan ponceau rrl; Pigment Ponceau R; Ponceau 2RL; Ponceau 2RX; Scarlet 2RB; Amacid scarlet 2r; Hispacid ponceau R; New Ponceau 4R; Paper Red HRR; Red For Lakes J; Kiton Ponceau 2R; Acid Ponceau 2RL; Acid Scarlet 2BN; Acid Scarlet 2RL; Acid Scarlet 2RN; Comacid scarlet 2r; Fenazo Scarlet 2R; Hexacol Ponceau MX; Kiton Scarlet 2RC; Red R; Hexacol Ponceau 2R; Hidacid Scarlet 2R; Lake Scarlet 2RBN; Acid Ponceau Special; Scarlet 2RL Bluish; Schultz no. 95; Calcocid Scarlet 2R; Xylidine Ponceau 3rs; Calcolake Scarlet 2R; Certicol Ponceau MXS; Naphthalene Scarlet R; Tertracid Ponceau 2R; Calcocid scarlet 2ril; C.I. 16150; Acid Leather Red KPR; Colacid Ponceau Special; Naphthazine Scarlet 2R; Acid Leather Red P2R; Ahcocid Fast Scarlet R; Edicol Supra Ponceau R; UNII-U3J3635T4U; Amacid Lake Scarlet 2R; Food Red No. 101; Acid Leather Scarlet IRW; D and C Red No. 5; D&C Red No. 5; Ponceau RR Type 8019; Naphthalene Lake Scarlet R; Acid Scarlet 2R For Lakes; Ponceau 2R Extra A Export; 1695 Red; Ponceau R (Biological stain); Ponceau 2R (Biological stain); Acid Scarlet 2R FOR Lakes Bluish; Ponceau Xylidine (Biological stain); C.I. Acid Red 26, Disodium Salt; C.I. 79; U3J3635T4U; 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-(2,4-xylylazo)-, disodium salt; MFCD00003897; Xylidine Ponceau 2R (C.l. 16150); Ponceaux 3R; D and C Red; Scarlet R (VAN); Tetracid Ponceau 2R; Disodium salt of 1-(2,4-xylylazo)-2-naphthol-3,6-disulfonic acid; Red No. 503; Cerven kysela 26 [Czech]; Red 101; Cerven kysela 26; 2,7-Naphthalenedisulfonic acid, 4-[2-(2,4-dimethylphenyl)diazenyl]-3-hydroxy-, sodium salt (1:2); CCRIS 164; Pas kwasowy 2 RL [Polish]; Pas kwasowy 2 RL; Cerven potravinarska 5 [Czech]; Cerven potravinarska 5; HSDB 2947; EINECS 223-178-3; NSC 10458; CI 16150; disodium 4-[(Z)-(2,4-dimethylphenyl)diazenyl]-3-hydroxy-2,7-naphthalenedisulfonate; disodium;4-[(E)-(2,4-dimethylphenyl)diazenyl]-3-hydroxynaphthalene-2,7-disulfonate; Ponceau Xylidine;; 2,7-Naphthalenedisulfonic acid, 4-(2-(2,4-dimethylphenyl)diazenyl)-3-hydroxy-, sodium salt (1:2); Disodium 1-(2,4-dimethylphenylazo)-2-hydroxynaphthalene-3,6-disulphonate; Ponceau 2 R; 1-Xylylazo-2-naphthol-3,6-disulfonic acid, disodium salt; 1-Xylylazo-2-naphthol-3,6-disulphonic acid, disodium salt; 1-(2,4-Xylylazo)-2-naphthol-3,6-disulfonic acid, disodium salt; 1-(2,4-Xylylazo)-2-naphthol-3,6-disulphonic acid, disodium salt; Disodium salt of 1-(2,4-xylylazo)-2-naphthol-3,6-disulphonic acid; Disodium (2,4-dimethylphenylazo)-2-hydroxynaphthalene-3,6-disulfonate; Disodium (2,4-dimethylphenylazo)-2-hydroxynaphthalene-3,6-disulphonate; 3-Hydroxy-4-(2,4-xylylazo)-2,7-naphthalenedisulfonic acid, disodium salt; 2,7-Naphthalenedisulfonic acid, 4-((2,4-dimethylphenyl)azo)-3-hydroxy-, disodium salt; 4-((2,4-Dimethylphenyl)azo)-3-hydroxy-2,7-naphthalenedisulfonic acid, disodium salt; 4-((2,4-Dimethylphenyl)azo)-3-hydroxy-2,7-naphthalenedisulphonic acid, disodium salt; disodium 4-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxynaphthalene-2,7-disulfonate; SCHEMBL6846278; CHEBI:82369; 6430AF; Ponceau Xylidine, analytical standard; 3-Hydroxy-4-(2,4-xylylazo)-2,7-naphthalenedisulphonic acid, disodium salt; AKOS000283082; AKOS015903398; AKOS024319210; NE18619; disodium 4-[(2,4-dimethylphenyl)diazenyl]-3-hydroxynaphthalene-2,7-disulfonate; Ponceau Xylidine, Dye content >=60 %; C.I. Acid Red 26, disodium salt (8CI); P0590; EN300-60349; Acid Red 26 100 microg/mL in Acetonitrile:Water; sodium (E)-4-((2,4-dimethylphenyl)diazenyl)-3-hydroxynaphthalene-2,7-disulfonate
|
| InChI |
1S/C18H16N2O7S2.2Na/c1-10-3-6-15(11(2)7-10)19-20-17-14-5-4-13(28(22,23)24)8-12(14)9-16(18(17)21)29(25,26)27;;/h3-9,21H,1-2H3,(H,22,23,24)(H,25,26,27);;/q;2*+1/p-2
|
 click to show the detail info of this DBT
click to show the detail info of this DBT