| Synonyms |
Click to Show/Hide the Synonyms of This DIG
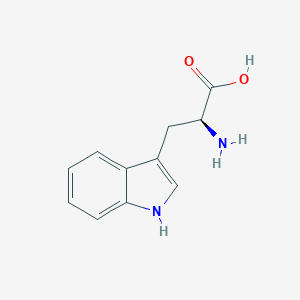
L-tryptophan; tryptophan; 73-22-3; L-Tryptophane; h-Trp-oh; (S)-Tryptophan; Tryptophane; Optimax; trofan; tryptacin; Ardeytropin; (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid; Pacitron; Indole-3-alanine; Kalma; L-beta-3-Indolylalanine; L-Tryptofan; L-Trp; L-(-)-Tryptophan; 3-Indol-3-ylalanine; Tryptan; Lyphan; Tryptophan (VAN); 1-beta-3-Indolylalanine; Tryptophan (H-3); Triptofano [Spanish]; Tryptophanum [Latin]; 1H-Indole-3-alanine; 1beta-3-Indolylalanine; 2-Amino-3-indolylpropanoic acid; triptofano; Tryptophanum; (S)-alpha-Amino-1H-indole-3-propanoic acid; Tryptophane [French]; Tryptophan, L-; (L)-TRYPTOPHAN; (-)-Tryptophan; alpha'-Amino-3-indolepropionic acid; Tryptophan [USAN:INN]; L-alpha-amino-3-indolepropionic acid; L-alpha-Aminoindole-3-propionic acid; Sedanoct; (S)-alpha-Aminoindole-3-propionic acid; 1H-Indole-3-alanine (VAN); EH 121; (S)-2-Amino-3-(3-indolyl)propionic acid; trp; Alanine, 3-indol-3-yl-; CCRIS 617; L-Alanine, 3-(1H-indol-3-yl)-; 1H-Indole-3-alanine, (S)-; alpha-Amino-3-indolepropionic acid, L-; HSDB 4142; (S)-alpha-amino-beta-(3-indolyl)-propionic acid; NCI-C01729; AI3-18478; UNII-8DUH1N11BX; MFCD00064340; Indole-3-propionic acid, alpha-amino-; 1H-Indole-3-propanoic acid, alpha-amino-, (S)-; Propionic acid, 2-amino-3-indol-3-yl-; CHEBI:16828; Lopac-T-0254; 8DUH1N11BX; (S)-alpha-Amino-beta-indolepropionic acid; CHEMBL54976; (S)-2-Amino-3-(1H-indol-3-yl)propanoic acid; DSSTox_CID_1419; DSSTox_RID_76152; L(-)-Tryptophan, 99%; DSSTox_GSID_21419; l-b-3-Indolylalanine; D-Trp-OH; CAS-73-22-3; Propionic acid, 2-amino-3-indol-3-yl; L-Tryptophan (9CI); Tryptophan (USP/INN); (S)-a-Amino-b-indolepropionic acid; (S)-a-Aminoindole-3-propionic acid; Alanine, 3-indol-3-yl; EINECS 200-795-6; NSC 13119; (2S)-2-amino-3-(1H-indol-3-yl)propanoate; trytophan; (S)-a-Amino-1H-indole-3-propanoic acid; TRP-01; 2-amino-3-indol-3-ylpropanoic acid; L-Trytophan; NSC-13119; 1qaw; L-Tryptophan,(S); L-Trp-OH; PubChem10984; 2a4m; H-L-Trp-OH; L-Tryptophan (JP17); S(-)-1-alpha-Aminoindole-3-propionic acid; Tryptophan (L-Tryptophan); Tryptophan, L- (8CI); bmse000050; bmse000868; bmse001017; Epitope ID:136043; EC 200-795-6; T 0254; SCHEMBL7328; 2-Amino-3-indolylpropanoate; (S)-(-)-2-Amino-3-(3-indolyl)propionic Acid; (S)-1H-Indole-3-alanine; Lopac0_001183; GTPL717; MLS001056750; DivK1c_000457; (s)-a-amino-b-indolepropionate; 151A3008-4CFE-40C9-AC0B-467EF0CB50EA; DTXSID5021419; (S)-a-Aminoindole-3-propionate; BDBM21974; HMS501G19; KBio1_000457; ZINC83315; 3-(1H-indol-3-yl)-L-Alanine; L-a-Amino-3-indolepropionic acid; NINDS_000457; alpha-Aminoindole-3-propionic acid; HMS3263N07; Pharmakon1600-01500600; ACT08662; HY-N0623; L-Tryptophan, Cell Culture Reagent; STR02722; (S)-alpha-Aminoindole-3-propionate; Tox21_201246; Tox21_300359; Tox21_501183; ANW-36308; HTS001390; NSC757373; s3987; (s)-alpha-amino-beta-indolepropionate; L-Tryptophan, Vetec(TM), 98.5%; (S)-a-Amino-1H-indole-3-propanoate; AKOS015854052; Indoe-3-propionic acid, alpha-amino-; 2-Chloro-5-(methylsulfonyl)benzoicacid; AM82273; CCG-205257; CS-W020011; DB00150; LP01183; MCULE-8004234494; NSC-757373; SB14997; SDCCGSBI-0051150.P002; IDI1_000457; NCGC00015994-01; NCGC00094437-01; NCGC00094437-02; NCGC00094437-03; NCGC00094437-04; NCGC00094437-08; NCGC00254424-01; NCGC00258798-01; NCGC00261868-01; (S)-alpha-Amino-1H-indole-3-propanoate; AC-17050; BP-13286; SMR000326686; DB-029986; L-Tryptophan, BioUltra, >=99.5% (NT); A7403; EU-0101183; (S)-Tryptophan 1H-Indole-3-alanine, (S)-; 73T223; C00078; D00020; L-Tryptophan, reagent grade, >=98% (HPLC); M02943; AB00373874_05; L-Tryptophan, Vetec(TM) reagent grade, >=98%; (S)-2-amino-3-(1H-Indol-3-yl)-propionic acid; Q181003; SR-01000075590; 4-(3-METHOXYANILINO)-4-OXOBUT-2-ENOICACID; SR-01000075590-1; F0001-2364; Z1245635763; L-Tryptophan, certified reference material, TraceCERT(R); Tryptophan, European Pharmacopoeia (EP) Reference Standard; UNII-0O72R8RF8A component QIVBCDIJIAJPQS-VIFPVBQESA-N; UNII-N7U7BXP2OI component QIVBCDIJIAJPQS-VIFPVBQESA-N; UNII-X9U7434L7A component QIVBCDIJIAJPQS-VIFPVBQESA-N; L-Tryptophan, United States Pharmacopeia (USP) Reference Standard; L-Tryptophan, from non-animal source, meets EP, JP, USP testing specifications, suitable for cell culture, 99.0-101.0%; L-Tryptophan, PharmaGrade, Ajinomoto, EP, JP, USP, Manufactured under appropriate GMP controls for pharma or biopharmaceutical production, suitable for cell culture
|
| InChI |
1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT