| Synonyms |
Click to Show/Hide the Synonyms of This DIG
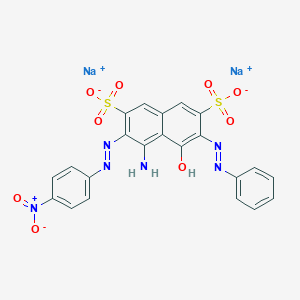
ACID BLACK 1; 1064-48-8; Naphthol Blue Black; Amido Black 10B; Acid black 2; Buffalo Black NBR; C.I. Acid Black 1; NIGROSINE; Amidoschwarz; 8005-03-6; Nigrosin; Acidal Black 10B; Amido black; C.I. Acid Black 1, disodium salt; Naphthol Blue Black B; Acidal Navy Blue 3BR; Acid Blue Black; Acid Blue-Black; C.I. 20470; Sandopel Blue P; Boruta Black A; Solar Blue Black; Acid Black H; Kiton Black HA; Acid Black BX; Blue Black SX; Eniacid Black SH; Kiton Blue SM; Acid Black BRX; Acid Black JVS; Aniline blue-black; Fenazo Blue Black; Azo Dark Blue S; Kiton Black 2B; Brasilan Black BS; Acid Black 4BN; Acid Black 4BNU; Bucacid Blue Black; Eniacid Black IVS; Hidacid Blue Black; Napthol Blue Black; Acid Black Base M; Acid Blue Black B; Azo Dark Blue HR; Azo Dark Blue SH; Calcocid Blue Black; Metamine Blue Black; Acid Black 10A; Acid Black 10B; Acid Black 12B; Acilan Black 10B; Blue Black 12B; Acid Black 10BA; Acid Black 10BN; CI Acid Black 1; Airedale Black 2BG; Colacid Black 10A; Leather Black 10B; Acid Blue Black BG; Amacid Black 10BR; Atul Acid Black BX; Wool Navy Blue 2B; Acid Brown 187; Hispacid Black 10B; Naphthol Black 12B; Amido Black 10 B; Comacid Blue Black B; Eriosin Blue Black B; Fast Sulfon Black BN; Naphtylamine Black 4B; D&C Black 1; Naphtocard Black 12B; Naphthol Blue Black G; Naphthol Blue Black T; Naphthalene Black 12B; Acid Blue Black 10B; Acid Leather Blue IGW; Java Blue Black 10B; Mitsui Acid Blue Black; UNII-SZT789770M; Naphthol Blue Black BC; Pontacyl Blue Black SX; Acid Leather Fast Blue Black G; Naphtylamine Black 10B; Atul Acid Black 10BX; Calcocid Blue Black 2R; Naphthalene Black 12BN; Naphthol Blue Black 6B; Diacid Blue Black 10B; Naphthol Blue Black CWC; Pontacyl Blue Black SXR; Tertracid Blue Black NB; Naphtylamine Black 10BN; CI 20470; Naphtol Blue Black 12B; Naphthylamine Black 10BR; D and C Black No. 1; Naphthol Blue Black 10B; Acid Leather Dark Blue G; D And C Black Number 1; Hastings Acid Black 10BG; Hastings Acid Black 10BR; Leather Fast Blue Black G; Vondamol Light Black 10B; Naphthazine Blue Black 6BN; Naphtylamine Black 10BR-CF; Azanol Fast Acid Black 10B; Kayaku Acid Blue Black 10B; MFCD00004017; Acid Blue Black Double 600; Amido Black 10B (C.I. 20470); Aminoschwarz 10B; SZT789770M; 2,7-Naphthalenedisulfonic acid, 4-amino-5-hydroxy-3-((4-nitrophenyl)azo)-6-(phenylazo)-, disodium salt; Nigrosine, pure, water soluble; Fast Sulon Black BN; Azo Dark Blue C 2B; CERN kysela 1 [Czech]; CERN kysela 1; CCRIS 6934; C.I. Acid Black 2; NSC 7820; EINECS 213-903-1; Nigrosine, pure, water soluble, high purity biological stain; Nigrosine B; Nigrosine WSB; HSDB 8004; CI Acid black 2; EINECS 280-105-8; MFCD00044681; Sodium 4-amino-5-hydroxy-3-(4-nitrophenylazo)-6-(phenylazo)naphthalene-2,7-disulphonate; ACIDBLACK2; Lurazol Deep Blue EB; Calco nigrosine O 2P; D&C Black No. 1; Nigrosine wl water soluble; SCHEMBL24037; 83006-55-7; SCHEMBL342498; DTXSID1020934; DTXSID1024415; C.I. Acid Black 1 (7CI); C22H14N6O9S2.2Na; CHEBI:86230; HSDB 1971; AKOS000282895; AKOS015903653; 4-Amino-5-hydroxy-3-(p-nitrophenylazo)-6-(phenylazo)-2,7-naphthalenedisulfonicaciddisodiumsalt; Naphthol Blue Black, Dye content 80 %; 4-Amino-5-hydroxy-3-((4-nitrophenyl)azo)-6-(phenylazo)-2,7-naphth- alenedisulfonic acid, disodium salt; 4-Amino-5-hydroxy-3-((4-nitrophenyl)azo)-6-(phenylazo)naphthalene-2,7-disulphonic acid, sodium salt; CI 50420; disodium 4-amino-5-hydroxy-3-[(E)-(4-nitrophenyl)diazenyl]-6-[(E)-phenyldiazenyl]naphthalene-2,7-disulfonate; Naphthol Blue Black, Dye content ~50 %; C.I. Acid Black 1, disodium salt (8CI); A0586; Q470900; J-001593; Q1876481; Amido Black 10B, 0.2% v/v solution in 5% acetic acid; Amido Black Staining Solution 2X, electrophoresis reagent; Naphthol Blue Black, BioReagent, suitable for electrophoresis; 2,7-Naphthalenedisulfonic acid, 4-amino-5-hydroxy-3-((4-nitrophenyl)azo)-6-(phenylazo)-, sodium salt; 2,7-Naphthalenedisulfonic acid, 4-amino-5-hydroxy-3-(2-(4-nitrophenyl)diazenyl)-6-(2-phenyldiazenyl)-, sodium salt (1:2); 3-(4-Nitrophenylazo)-4-amino-5-oxo-6-(2-phenylhydrazono)-5,6-dihydro-2,7-naphthalenedisulfonic acid disodium salt; Sodium 4-amino-5-hydroxy-3-((4-nitrophenyl)diazenyl)-6-(phenyldiazenyl)naphthalene-2,7-disulfonate; sodium 4-amino-5-hydroxy-3-((E)-(4-nitrophenyl)diazenyl)-6-((E)-phenyldiazenyl)naphthalene-2,7-disulfonate
|
| InChI |
1S/C22H16N6O9S2.2Na/c23-19-18-12(11-17(39(35,36)37)21(22(18)29)27-24-13-4-2-1-3-5-13)10-16(38(32,33)34)20(19)26-25-14-6-8-15(9-7-14)28(30)31;;/h1-11,29H,23H2,(H,32,33,34)(H,35,36,37);;/q;2*+1/p-2
|
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT