| Synonyms |
Click to Show/Hide the Synonyms of This API
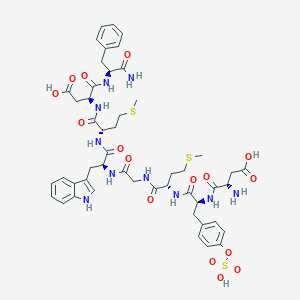
Sincalide; 25126-32-3; CCK-8; SQ 19844; UNII-M03GIQ7Z6P; cholecystokinin C-terminal octapeptide; CHEMBL1121; M03GIQ7Z6P; H-Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2; 3-10-Caerulein, 5-L-methionine-; MFCD00079849; Kinevac; cholecystokinin 8; DSSTox_CID_28543; DSSTox_RID_82815; DSSTox_GSID_48617; (Tyr[SO3H]27)Cholecystokinin fragment 26-33 Amide; Sincalidum; Sincalida; Syncalide; (3S,6S,9S,15S,18S,21S)-9-((1H-indol-3-yl)methyl)-21-amino-3-(((S)-1-amino-1-oxo-3-phenylpropan-2-yl)carbamoyl)-6,15-bis(2-(methylthio)ethyl)-5,8,11,14,17,20-hexaoxo-18-(4-(sulfooxy)benzyl)-4,7,10,13,16,19-hexaazatricosanedioic acid; CAS-25126-32-3; Human CCK-8; CCK-8 (sulphated); Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2; Sincalidum [INN-Latin]; Sincalida [INN-Spanish]; Cholecystokinin octapeptide; Pancreozymin; SQ-19844; Sincalide [USAN:USP:INN:BAN]; NCGC00183278-01; NCGC00183363-01; C49H62N10O16S3; EINECS 246-639-0; Kinevac (TN); Sincalide (USAN/INN); Caerulein, 1-de(5-oxo-L-proline)-2-de-L-glutamine-5-L-methionine-; CCK C-terminal octapeptide; Cholecystokinin-pancreozymin; cholecystokinin fragment 26-33 amide (sulphated); GTPL864; SCHEMBL122365; CCK-8(SO3); DTXSID7048617; BDBM21147; CHEBI:135946; Pancreozymin C-terminal octapeptide; [125I]CCK-8; 1-De(5-oxo-L-proline)-2-de-L-glutamine-5-L-methioninecaerulein; HY-P0093; Tox21_112955; Tox21_113481; Cholecystokinin Octapeptide, sulfated; CORALYNECHLORIDEHYDRATE,98+%; AKOS016340423; CS-5963; DB09142; HS-2026; NCGC00167273-01; L-alpha-Aspartyl-O-sulfo-L-tyrosyl-L-methionylglycyl-L-tryptophyl-L-methionyl-L-alpha-aspartyl-L-phenylalaninamide; L-Aspartyl-L-tyrosyl-L-methionylglycyl-L-tryptophyl-L-methionyl-L-aspartylphenyl-L-alaninamide hydrogen sulfate (ester); O411; Cholecystokinin, CCK Octapeptide (26-33); D05845; Asp26-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-PheNH2; 126S323; A817659; Cholecystokinin Octapeptide (sulfated) ammonium salt; Q7521885; (3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-amino-3-formamidopropanoic acid]-3-[4-(sulfooxy)phenyl]propanamido]-4-(methylsulfanyl)butanamido]acetamido}-3-(1H-indol-3-yl)propanamido]-4-(methylsulfanyl)butanamido]-3-{[(1S)-1-carbamoyl-2-phenylethyl]carbamoyl}propanoic acid; (3S)-3-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-4-hydroxy-4-oxobutanoyl]amino]-3-(4-sulfooxyphenyl)propanoyl]amino]-4-methylsulfanylbutanoyl]amino]acetyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-4-methylsulfanylbutanoyl]amino]-4-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid; (3S)-3-amino-4-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-4-(methylthio)-1-oxobutan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-4-(methylth; (3S)-3-amino-4-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-4-methyls; (3S)-3-azanyl-4-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-azanyl-1-oxidanylidene-3-phenyl-propan-2-yl]amino]-4-oxidanyl-1,4-bis(oxidanylidene)butan-2-yl]amino]-4-methylsulfanyl-1-oxidanylidene-butan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxidanyl; (3S,6S,9S,15S,18S,21S)-9-((1H-indol-3-yl)methyl)-21-amino-3-(((S)-1-amino-1-oxo-3-phenylpropan-2-yl)carbamoyl)-6,15-bis(2-(methylthio)ethyl)-5,8,11,14,17,20-hexaoxo-18-(4-(sulfooxy)benzyl)-4,7,10,13,16,19-hexaazatricosane-1,23-dioic acid; (3S,6S,9S,15S,18S,21S)-9-((1H-indol-3-yl)methyl)-21-amino-3-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)-6,15-bis(2-(methylthio)ethyl)-5,8,11,14,17,20-hexaoxo-18-(4-(sulfooxy)benzyl)-4,7,10,13,16,19-hexaazatricosane-1,23-dioic acid
|
| InChI |
1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1
|
 click to show the detail info of this DFM
click to show the detail info of this DFM