Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00122) | |||||
|---|---|---|---|---|---|
| Name |
Chlorothiazide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
chlorothiazide; 58-94-6; Diuril; Chlorothiazid; Chlorthiazide; Chlotride; Thiazide; Chlortiazid; Chlorosal; Chlorurit; Saluretil; Warduzide; Alurene; Clotride; Diuresal; Diurilix; Diurite; Diutrid; Salisan; Salunil; Saluric; Yadalan; Flumen; Minzil; Urinex; Neo-Dema; Sk-chlorothiazide; Chlorothiazidum; Aldoclor; Clorotiazida; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-, 1,1-dioxide; 6-Chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; component of Aldoclor; 6-Chloro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; NSC 25693; UNII-77W477J15H; MFCD00058576; Diuril (TN); MLS000028398; CHEBI:3640; Chlorthiazid; Chlorthiazidum; 6-chloro-1,1-dioxo-4H-1$l^{6},2,4-benzothiadiazine-7-sulfonamide; NSC25693; NSC-25693; 77W477J15H; Chlorothiazide, 98%; CAS-58-94-6; NCGC00015242-04; Clorotiazide; Chloriazid; Chlrosal; SMR000058429; 6-chloro-4H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; Clorotiazide [DCIT]; Diuril Boluses; DSSTox_CID_2800; DSSTox_RID_76733; DSSTox_GSID_22800; Diuril Boluses, Veterinary; C7H6ClN3O4S2; Clorotiazida [INN-Spanish]; Chlorothiazidum [INN-Latin]; chlorothiazid-; CCRIS 5999; HSDB 3030; SR-01000075604; EINECS 200-404-9; 6-chloro-1,1-dioxo-2H-1,2,4-benzothiadiazine-7-sulfonamide; 6-chloro-1,1-dioxo-2H-1?^{6},2,4-benzothiadiazine-7-sulfonamide; Chlorothiazide (JAN/USP/INN); Chlorothiazide [USP:INN:BAN]; Prestwick_56; Diupres (Salt/Mix); Aldoclor (Salt/Mix); Spectrum_000134; Prestwick0_000251; Prestwick1_000251; Prestwick2_000251; Prestwick3_000251; Spectrum2_000154; Spectrum3_000342; Spectrum4_000280; Spectrum5_001446; Lopac-C-4911; CHEMBL842; C 4911; cid_2720; Lopac0_000254; SCHEMBL22329; BSPBio_000062; BSPBio_002003; KBioGR_000780; KBioSS_000594; BIDD:GT0635; DivK1c_000675; SPECTRUM1500180; SPBio_000288; SPBio_002281; BPBio1_000070; GTPL4835; CHEMBL3392493; DTXSID0022800; BDBM39351; Chlorothiazide, thiazide diuretic; HMS502B17; KBio1_000675; KBio2_000594; KBio2_003162; KBio2_005730; KBio3_001223; NINDS_000675; HMS1568D04; HMS1920K15; HMS2091C18; HMS2095D04; HMS2232N22; HMS3259K15; HMS3260D10; HMS3370A15; HMS3655M13; HMS3712D04; Pharmakon1600-01500180; BCP24474; HY-B0224; ZINC3872055; Tox21_110107; Tox21_200972; Tox21_500254; WLN: T66 BSWM ENJ HG ISZW; 6-chloro-4H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide; CCG-38953; NSC756682; AKOS015896601; AKOS024319450; Tox21_110107_1; DB00880; LP00254; MCULE-3497410863; NC00500; NSC-756682; SDCCGSBI-0050242.P005; IDI1_000675; NCGC00015242-01; NCGC00015242-02; NCGC00015242-03; NCGC00015242-05; NCGC00015242-06; NCGC00015242-07; NCGC00015242-08; NCGC00015242-09; NCGC00015242-10; NCGC00015242-12; NCGC00015242-13; NCGC00015242-19; NCGC00091042-01; NCGC00091042-02; NCGC00091042-03; NCGC00091042-04; NCGC00091042-05; NCGC00258525-01; NCGC00260939-01; AC-18732; AS-11760; SBI-0050242.P004; AB0010688; EU-0100254; FT-0664953; Hydrochlorothiazide impurity, chlorothiazide-; SW219268-1; Chlorothiazide 100 microg/mL in Acetonitrile; C07461; D00519; AB00051940-04; AB00051940_05; AB00051940_06; 102632-EP2270011A1; 102632-EP2272841A1; 102632-EP2277879A1; 102632-EP2298776A1; 102632-EP2301936A1; Q2603363; SR-01000075604-1; SR-01000075604-3; SR-01000075604-5; W-105353; BRD-K88682005-001-05-9; BRD-K88682005-001-07-5; Z1691545266; 6-Chloro-7-sulfamoyl-2H-1,4-benzothiadiazine 1,1-dioxide; 6-Chloro-2H-1,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-chloro-4H-1,2,4-benzathiadiazine-7-sulfonamide-1,1-dioxide; Chlorothiazide, European Pharmacopoeia (EP) Reference Standard; 2H-1,4-Benzothiadiazine-7-sulfonamide, 6-chloro-, 1,1-dioxide; 6-chloro-1,1-dioxo-2H-1$l^{6},2,4-benzothiadiazine-7-sulfonamide; 6-chloro-1,1-dioxo-2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide; 6-chloro-1,1-dioxo-4H-1$l^{6,2,4-benzothiadiazine-7-sulfonamide; 6-chloro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide; Chlorothiazide, United States Pharmacopeia (USP) Reference Standard; Chlorothiazide, Pharmaceutical Secondary Standard; Certified Reference Material
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Congestive heart failure | ICD-11: BD10 | [1] | ||
| PubChem CID | |||||
| Formula |
C7H6ClN3O4S2
|
||||
| Canonical SMILES |
C1=C2C(=CC(=C1Cl)S(=O)(=O)N)S(=O)(=O)N=CN2
|
||||
| InChI |
1S/C7H6ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-3H,(H,10,11)(H2,9,12,13)
|
||||
| InChIKey |
JBMKAUGHUNFTOL-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=2720"></iframe>
|
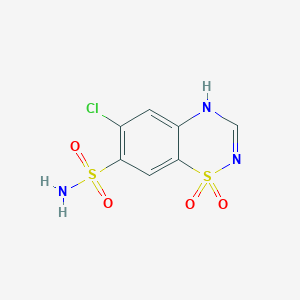 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 295.7 | Topological Polar Surface Area | 135 | |
| XlogP | -0.2 | Complexity | 532 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 1 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Chlorothiazide 250 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Sodium lauryl sulfate; Magnesium stearate; Polysorbate 80; Silicon dioxide; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | West-Ward Pharmaceticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium lauryl sulfate; Magnesium stearate; Croscarmellose sodium; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Avera McKennan Hospital; Mylan Pharamceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Chlorothiazide 500 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Anhydrous lactose; Sodium lauryl sulfate; Magnesium stearate; Polysorbate 80; Silicon dioxide; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | West-Ward Pharmaceticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium lauryl sulfate; Magnesium stearate; Croscarmellose sodium; Silicon dioxide; Cellulose, microcrystalline
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Mylan Pharamceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
