Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00707) | |||||
|---|---|---|---|---|---|
| Name |
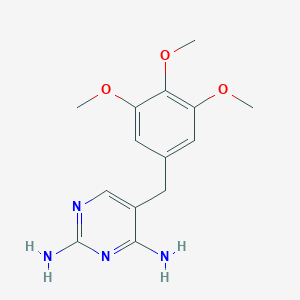
Trimethoprim
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
trimethoprim; 738-70-5; Proloprim; Trimpex; Trimetoprim; Bactramin; Monotrim; Monotrimin; Trimopan; 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine; Monoprim; Syraprim; Trimanyl; Wellcoprim; Triprim; Uretrim; 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine; Trimethoprime; Trimethoprimum; NIH 204; Primsol; 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine; component of Bactrim; NSC-106568; BW 56-72; 2,4-Pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]-; Polytrim; TCMDC-125538; 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine; UNII-AN164J8Y0X; 5-[(3,4,5-Trimethoxyphenyl)methyl]-2,4-pyrimidinediamine; NSC 106568; 5-(3,4,5-Trimethoxybenzyl)-2,4-diaminopyrimidine; CHEBI:45924; CHEMBL22; Trimpex (TN); Abaprim; MFCD00036761; Apo-Sulfatrim; BW 5672; BW-56-72; Pyrimidine, 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-; MLS000079023; AN164J8Y0X; Briscotrim; Novotrimel; Streptoplus; Sulfoxaprim; Trimethioprim; Urobactrim; Wellcoprin; Anitrim; Antrima; Antrimox; Bacidal; Bacticel; Bactoprim; Bencole; Bethaprim; Biosulten; Chemotrin; Colizole; Conprim; Cotrimel; Duocide; Esbesul; Espectrin; Euctrim; Exbesul; Fermagex; Fortrim; Ikaprim; Kombinax; Lagatrim; Lastrim; Metoprim; Pancidim; Protrin; Resprim; Salvatrim; Setprin; Sinotrim; Sugaprim; Sulfamar; Sulthrim; Sultrex; Trimexol; Trimezol; Trimono; Trisulcom; Trisulfam; Trisural; Utetrin; Velaten; Xeroprim; Zamboprim; 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)-methyl)-; 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)methyl)-; Bacdan; Bacide; Deprim; Omstat; Purbal; Roubac; Roubal; Stopan; Toprim; Trisul; Trimpex 200; Co-Trimoxizole; Lagatrim Forte; Septrin Forte; Alcorim-F; Colizole DS; Septrin S; Septrin DS; Smz-Tmp; NSC106568; Trimez-IFSA; Trimethoprim, 98%; U-Prin; component of Septra; NCGC00016055-05; Trimethopriom; 5-((3,4,5-Trimethoxyphenyl)methyl)-2,4-pyrimidinediamine; Bactifor; CAS-738-70-5; Dosulfin; Instalac; SMR000035999; Trimetoprim [DCIT]; Trimogal; Lescot; Tiempe; Trimetoprim [Polish]; Resprim Forte; Uro-D S; DSSTox_CID_3712; Tmp Smx; DSSTox_RID_77158; DSSTox_GSID_23712; 5-(3,4,5-Trimethoxy-benzyl)-pyrimidine-2,4-diamine; Trimethoprime [INN-French]; Trimethoprimum [INN-Latin]; Trimetoprima [INN-Spanish]; 2,4-Diamino-5-(3',4',5'-trimethoxybenzyl)pyrimidine; Bacterial [Antibiotic]; NIH 204 (VAN); Proloprim (TN); WR 5949; CCRIS 2410; HSDB 6781; SR-01000075652; EINECS 212-006-2; 5-(3, 4, 5-Trimethoxybenzyl)-2, 4-pyrimidinediamine; BRN 0625127; Trimethoprim (JAN/USP/INN); 5-{[3,4,5-tris(methyloxy)phenyl]methyl}pyrimidine-2,4-diamine; AI3-52594; B-Lock; KUC103659N; Trimethoprim,(S); Prestwick_485; KSC-4-158; Trimethoprim (TMP); Bactrim (Salt/Mix); AZT + TMP/SMX (mixture) combination; Spectrum_000167; Tocris-0650; Trimethoprim [USAN:USP:INN:BAN:JAN]; Opera_ID_1760; Prestwick0_000208; Prestwick1_000208; Prestwick2_000208; Prestwick3_000208; Spectrum2_000937; Spectrum3_000643; Spectrum4_000372; Spectrum5_001559; Lopac-T-7883; Epitope ID:119684; UPCMLD-DP132; T 7883; Lopac0_001271; Oprea1_495058; SCHEMBL24506; BSPBio_000195; BSPBio_002245; KBioGR_000863; KBioSS_000647; 5-25-13-00429 (Beilstein Handbook Reference); MLS001201740; MLS002303068; MLS002548881; ARONIS24118; BIDD:GT0190; DivK1c_000589; SPECTRUM1500595; SPBio_000874; SPBio_002116; BPBio1_000215; DTXSID3023712; UPCMLD-DP132:001; BDBM18069; GTPL10931; HMS501N11; KBio1_000589; KBio2_000647; KBio2_003215; KBio2_005783; KBio3_001465; NIH-204; Trimethoprim, >=98% (HPLC); NINDS_000589; 2,4,5-trimethoxybenzyl)pyrimidine; HMS1568J17; HMS1921I03; HMS2090D14; HMS2092A10; HMS2095J17; HMS2230L06; HMS3259I11; HMS3263P04; HMS3371O18; HMS3652E03; HMS3712J17; Pharmakon1600-01500595; 2,4,5-trimethoxyphenzyl)pyrimidine; ALBB-028968; BCP12148; HY-B0510; ZINC6627681; Tox21_110291; Tox21_200157; Tox21_501271; ANW-42623; BBL005584; CCG-40335; KM1575; NSC752719; NSC757370; SBB080617; STK177322; STL455117; AKOS001650069; Tox21_110291_1; AC-8427; BW-5672; DB00440; KS-1145; LP01271; MCULE-6670904388; NC00483; NSC-752719; NSC-757370; SDCCGSBI-0051237.P004; IDI1_000589; SMP2_000262; NCGC00016055-01; NCGC00016055-02; NCGC00016055-03; NCGC00016055-04; NCGC00016055-06; NCGC00016055-07; NCGC00016055-08; NCGC00016055-09; NCGC00016055-10; NCGC00016055-11; NCGC00016055-12; NCGC00016055-13; NCGC00016055-14; NCGC00016055-16; NCGC00016055-17; NCGC00016055-27; NCGC00024707-01; NCGC00024707-03; NCGC00024707-04; NCGC00024707-05; NCGC00024707-06; NCGC00024707-07; NCGC00024707-08; NCGC00257711-01; NCGC00261956-01; AK154007; ST024737; SY031734; Trimethoprim/sulfamethoxazole (commercial); SBI-0051237.P003; AB0007348; DB-055812; Trimethoprim 100 microg/mL in Acetonitrile; 2, 5-[(3,4,5-trimethoxyphenyl)methyl]-; AB00052118; BB 0258034; EU-0101271; FT-0601630; FT-0675578; FT-0675579; FT-0675580; SW196690-3; C01965; D00145; J10046; Trimethoprim, crystallized, >=99.0% (HPLC); WLN: T6N CNJ BZ DZ E1R CO1 DO1 EO1; 5-(3,5-Trimethoxybenzyl)-2,4-diaminopyrimidine; AB00052118-30; AB00052118-32; AB00052118_33; AB00052118_34; Trimethoprim, VETRANAL(TM), analytical standard; 738T705; Formulated trimethoprim (NSC 106568) in ethanol; Q422665; 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine; 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-pyrimidine; Pyrimidine,4-diamino-5-(3,4,5-trimethoxybenzyl)-; SR-01000075652-1; SR-01000075652-3; SR-01000075652-6; W-104441; 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine #; BRD-K07208025-001-06-5; SR-01000075652-10; 2-amino-5-(3,4,5-trimethoxybenzyl)-4-pyrimidinylamine; F0914-5266; Trimethoprim, certified reference material, TraceCERT(R); Z1522566629; 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4(1H,3H)-diimine; Trimethoprim, British Pharmacopoeia (BP) Reference Standard; Trimethoprim, European Pharmacopoeia (EP) Reference Standard; Trimethoprim, United States Pharmacopeia (USP) Reference Standard; Trimethoprim, Pharmaceutical Secondary Standard; Certified Reference Material
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Infectious cystitis | ICD-11: GC00 | [1] | ||
| PubChem CID | |||||
| Formula |
C14H18N4O3
|
||||
| Canonical SMILES |
COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N
|
||||
| InChI |
1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18)
|
||||
| InChIKey |
IEDVJHCEMCRBQM-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5578"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 290.32 | Topological Polar Surface Area | 106 | |
| XlogP | 0.9 | Complexity | 307 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 5 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Trimethoprim 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 4 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Anhydrous lactose; Sodium lauryl sulfate; Magnesium stearate; Silicon dioxide; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Actavis Pharma; AvPAK; Carilion Materials Management; Physicians Total Care | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Magnesium stearate; Water; Silicon dioxide; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | RemedyRepack | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Magnesium stearate; Water; Silicon dioxide; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | AvKARE; Lupin Pharmaceuticals; Novel Laboratories | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium stearate; Dibasic calcium phosphate dihydrate; Silicon dioxide; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Mayne Pharma; Teva Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
