| Synonyms |
Click to Show/Hide the Synonyms of This API
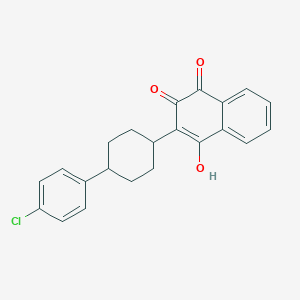
ATOVAQUONE; 95233-18-4; Mepron; Wellvone; Acuvel; Atavaquone; 566C80; Mepron (antipneumocystic); 94015-53-9; cis-Atovaquone; BW 566C; 566C; C22H19ClO3; 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxynaphthalene-1,2-dione; Atovaquone (Atavaquone); 137732-39-9; 2-(trans-4-(p-Chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone; UNII-Y883P1Z2LT; UNII-F1W7QUV0KI; F1W7QUV0KI; 2-(4-(4-Chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone; 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone; trans-2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione; 2-(TRANS-4-(4-CHLOROPHENYL)CYCLOHEXYL)-3-HYDROXY-1,4-NAPHTHALENEDIONE; CHEMBL519462; Y883P1Z2LT; CHEBI:575568; 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxynaphthalene-1,4-dione; NCGC00016961-01; CAS-95233-18-4; 2-[trans-4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione; DSSTox_CID_2629; DSSTox_RID_76664; DSSTox_GSID_22629; 2-[trans-4-(p-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone; 1,4-Naphthalenedione, 2-[cis-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-; 2-((1r,4r)-4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione; DRG-0084; BW 566C-80; Mepron (TN); BW-A 566C; HSDB 7083; SR-05000001438; BW-566C-80; CRL-8131 & Atovaquone; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone; Atovaquone & Interleukin 12; 2-(4-(4-Chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-naphthalene-1,4-dione; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxynaphthalene-1,4-dione; cis-2-(4-(4-Chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone; Cis-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone; 1,4-Naphthalenedione, 2-(cis-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-; 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione; trans-2-(4-(4-chlorophenyl) cyclohexyl)-3-hydroxynaphthalene-1,4-dione; ATO & IL-12; Atovaquone [USAN:USP:INN:BAN]; Atovaquone-[d5]; BW 566C80; 1329792-63-3; Spectrum_001743; SpecPlus_000686; Prestwick0_000534; Prestwick1_000534; Prestwick2_000534; Prestwick3_000534; Spectrum2_001665; Spectrum3_000991; Spectrum4_001117; Spectrum5_001382; Atovaquone EP Impurity B; SCHEMBL21694; SCHEMBL21695; Atovaquone (JAN/USP/INN); BSPBio_000547; BSPBio_002681; KBioGR_001594; KBioSS_002223; Atovaquone Related Compound A; MLS002153863; BIDD:GT0849; DivK1c_006782; SCHEMBL637069; SPECTRUM1504210; SPBio_001849; SPBio_002468; BPBio1_000603; CHEMBL222334; CHEMBL471792; GTPL9695; NAP016; SCHEMBL1542719; SCHEMBL1649508; SCHEMBL9975142; SCHEMBL9975229; Atovaquone, >=98% (HPLC); DTXSID7022629; CHEBI:95346; KBio1_001726; KBio2_002223; KBio2_004791; KBio2_007359; KBio3_001901; DTXSID20916694; BDBM192009; HMS1569L09; HMS1922F19; HMS2089M14; HMS2093C10; HMS2096L09; HMS2235N08; HMS3369N09; HMS3651N20; HMS3713L09; Pharmakon1600-01504210; AMY15339; BCP09477; Tox21_110714; 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy-naphthalene-1,2-dione; Atovaquone related compound A [USP]; CCG-39090; FD7252; MFCD00889188; NSC759582; s3079; STK636160; trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone; ZINC12504271; 1,4-Naphthalenedione, 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-, trans-; 1,4-Naphthalenedione, 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-dihydronaphthalene-1,4-dione; AKOS005567953; AKOS015895691; AKOS015961933; Tox21_110714_1; ZINC100017856; ZINC100345537; ZINC116473771; ZINC299873031; BW-556C-80; CCG-220534; DB01117; MCULE-7318126574; NSC-759582; NCGC00016961-02; NCGC00016961-03; NCGC00016961-04; NCGC00016961-06; NCGC00016961-07; NCGC00095113-01; NCGC00095113-02; AC-30251; AK544285; AS-12809; Atovaquone 100 microg/mL in Acetonitrile; HY-13832; SMR001233220; SBI-0052893.P002; AB0012456; AB0107187; AB0211463; AB00513855; FT-0602868; SW219222-1; A13708; C06835; D00236; J90007; 78668-EP2307343A1; AB00053222-03; AB00053222_04; AB00053222_05; 233A184; Q418179; SR-05000001438-1; SR-05000001438-2; SR-05000001438-4; SR-05000001438-5; Z1541632806; 2-[4-(4-chlorophenyl)cyclohexy]-3-hydroxy-1,4-naphthoquinone; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-napthoquinone; 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1.4-naphthoquinone; 2-Hydroxy-3-[4-(4-chlorophenyl)cyclohexyl]-1,4-naphthoquinone; 3-[4-(p-chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthoquinone; 1,4-Naphthalenedione, 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-; 2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxynaphthoquinone, trans-; 3-[4-(4-Chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthalenedione; 1,4-Naphthalenedione, 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy; 2-(cis-4-(4-Chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione; cis -2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione; 2-hydroxy-3-[(1r,4r)-4-(4-chlorophenyl)cyclohexyl]-1,4-dihydronaphthalene-1,4-dione
|
 click to show the detail info of this DFM
click to show the detail info of this DFM