| General Information of API (ID: D00883) |
| Name |
Binimetinib
|
| Synonyms |
Click to Show/Hide the Synonyms of This API
Binimetinib; 606143-89-9; MEK162; ARRY-162; ARRY-438162; 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide; MEK-162; Mektovi; ARRY 162; ARRY 438162; UNII-181R97MR71; Binimetinib (MEK-162); MEK162 (ARRY-162, ARRY-438162); NVP-MEK162; Binimetinib (MEK162, ARRY-162, ARRY-438162); 181R97MR71; 6-(4-bromo-2-fluoroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide; 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide.; 5-(4-Bromo-2-fluoroanilino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide; Binimetinib [USAN:INN]; binimetinibum; Mektovi (TN); ARRY-162; ARRY-438162; MEK 162; ARRY 162; ARRY 438162; Mek162, Binimetinib; MEK162(Binimetinib); Binimetinib (JAN/USAN); cc-455; MLS006011180; C17H15BrF2N4O3; SCHEMBL570088; GTPL7921; CHEMBL3187723; MEK162 (Arry-162); AMY9056; AOB2072; DTXSID70209422; QCR-138; ARRY-162,MEK-162; CHEBI:145371; HMS3652J14; HMS3747G09; BCP06780; EX-A1024; MFCD22124525; NSC764042; NSC788187; NSC799361; s7007; ZINC38460704; AKOS026750517; CCG-269133; CS-0627; DB11967; NSC-764042; NSC-788187; NSC-799361; SB16501; NCGC00345804-01; NCGC00345804-10; 1073666-70-2; 6-(4-bromo-2-fluorophenylamino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-3H-benzo[d]imidazole-5-carboxamide; AC-29023; AK175886; AS-16706; DA-35030; HY-15202; SMR004702949; cas:606143-89-9;MEK162; AB0095018; FT-0697088; SW219910-1; Y1468; A11493; D10604; W-5894; Binimetinib;MEK-162; ARRY-162;ARRY-438162; J-516581; Q19903515; 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-1,3-benzodiazole-6-carboxamide; 5-[(4-Bromo-2-Fluorophenyl)Amino]-4-Fluoro-N-(2-Hydroxyethoxy)-1-Methyl-1H-Benzimidazole-6-Carboxami; 6-(4-bromo-2-fluorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxyethyoxy)-amide; 6-[(4-bromo-2-fluorophenyl)amino]-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide; N-(2-hydroxyethoxy)-4-fluoro-5-(2-fluoro-4-bromophenylamino)-1-methyl-1H-benzoimidazole-6-carboxamide; QO7
|
| Clinical Status |
Approved
|
| PubChem CID |
|
| Formula |
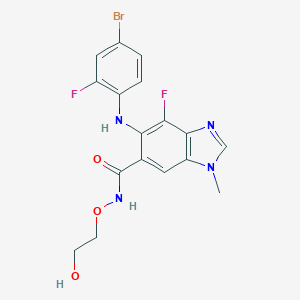
C17H15BrF2N4O3
|
| Canonical SMILES |
CN1C=NC2=C1C=C(C(=C2F)NC3=C(C=C(C=C3)Br)F)C(=O)NOCCO
|
| InChI |
1S/C17H15BrF2N4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26)
|
| InChIKey |
ACWZRVQXLIRSDF-UHFFFAOYSA-N
|
|
Click to Show/Hide the Molecular Data (Structure/Property) of This API
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=10288191"></iframe>
|
 |
|
3D MOL
|
2D MOL
|
| Physicochemical Properties |
Molecular Weight |
441.2 |
Topological Polar Surface Area |
88.4 |
| XlogP |
3.1 |
Complexity |
521 |
| Heavy Atom Count |
27 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
|
|
|
|
|
|
 click to show the detail info of this DFM
click to show the detail info of this DFM

