| Synonyms |
Click to Show/Hide the Synonyms of This API
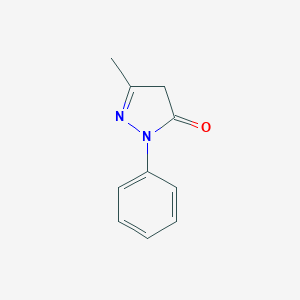
edaravone; 89-25-8; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE; 1-Phenyl-3-methyl-5-pyrazolone; Norphenazone; Radicut; MCI-186; Methylphenylpyrazolone; Developer Z; Norantipyrine; Phenylmethylpyrazolone; C.I. Developer 1; Phenyl methyl pyrazolone; 3-Methyl-1-phenyl-1H-pyrazol-5(4H)-one; Radicava; 1-Phenyl-3-methyl-5-oxo-2-pyrazoline; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one; 3H-Pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl-; 5-methyl-2-phenyl-4H-pyrazol-3-one; 1-Phenyl-3-methylpyrazolone; 1-Phenyl-3-methylpyrazolone-5; 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 3-Methyl-1-phenylpyrazol-5-one; 3-Methyl-1-phenyl-2-pyrazoline-5-one; NCI-C03952; 2-Pyrazolin-5-one, 3-methyl-1-phenyl-; 5-Pyrazolone, 3-methyl-1-phenyl-; Edaravone (MCI-186); 1-Fenyl-3-methyl-2-pyrazolin-5-on; MFCD00003138; 3-methyl-1-phenyl-4,5-dihydro-1H-pyrazol-5-one; CHEBI:31530; NSC-2629; NSC-26139; Antipyrine related compound a; MLS000069602; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE (MCI-186); CI Developer 1; NCGC00164015-01; SMR000059020; DSSTox_CID_1130; DSSTox_RID_75961; DSSTox_GSID_21130; Monopyrazolone; WLN: T5NMV DHJ BR& E1; 1-Phenyl-3-methyl-2-pyrazolin-5-on; CAS-89-25-8; CCRIS 512; Radicut (TN); 3-Methyl-1-phenyl-2-pyrazolin-5-one, 99%; HSDB 4102; 3H-Pyrazol-3-one,4-dihydro-5-methyl-2-phenyl-; SR-01000000135; 1-Fenyl-3-methyl-2-pyrazolin-5-on [Czech]; EINECS 201-891-0; BRN 0609575; AI3-03557; Radicava (TN); (MCI-186); CDS1_000986; PubChem13301; Spectrum_000267; Tocris-0786; MCI-186; Edaravone; Edaravone [USAN:INN]; Maybridge1_005738; Opera_ID_1057; Spectrum2_001574; Spectrum3_000971; Spectrum4_001091; Spectrum5_001217; M0687; EC 201-891-0; SCHEMBL4704; BSPBio_001235; BSPBio_002601; KBioGR_000575; KBioGR_001502; KBioSS_000575; KBioSS_000747; AE-641/00371017; MLS001146878; MLS002415675; MLS006011753; DivK1c_001018; DivK1c_002026; SPECTRUM1503635; SPBio_001508; CHEMBL290916; Edaravone (USAN/JP17/INN); DTXSID9021130; BCBcMAP01_000127; HMS503K17; HMS557M18; KBio1_001018; KBio2_000575; KBio2_000747; KBio2_003143; KBio2_003315; KBio2_005711; KBio2_005883; KBio3_001029; KBio3_001030; KBio3_001821; NSC2629; NINDS_001018; BCPP000246; Bio1_000438; Bio1_000927; Bio1_001416; Bio2_000448; Bio2_000928; HMS1362M17; HMS1792M17; HMS1990M17; HMS2234M19; HMS3266F04; HMS3403M17; HMS3411L05; HMS3654L15; HMS3675L05; HMS3884A11; Pharmakon1600-01503635; ACT07289; BCP26336; HY-B0099; NSC26139; Tox21_112077; Tox21_201747; Tox21_302819; BBL011741; BDBM50200541; CCG-39352; NSC758622; s1326; STK201315; ZINC18203737; 3-methyl-1-phenyl-2-pyrazolin-5one; AKOS000313817; Tox21_112077_1; AC-4745; BCP9000635; CS-1832; DB12243; NE10266; NSC-758622; SB19128; IDI1_001018; IDI1_002203; 1-PEHNYL-3-METHYL-5-PYRAZALONE; NCGC00018218-01; NCGC00018218-02; NCGC00018218-03; NCGC00018218-04; NCGC00018218-05; NCGC00018218-06; NCGC00018218-07; NCGC00018218-08; NCGC00018218-10; NCGC00018218-17; NCGC00022665-02; NCGC00022665-04; NCGC00022665-05; NCGC00022665-06; NCGC00256515-01; NCGC00259296-01; AK128848; ST012744; SBI-0051836.P002; DB-002517; AM20060748; FT-0608243; SW148216-2; 5-methyl-2-phenyl-2,4-dihydro-3-pyrazolone; 5-methyl-2-phenyl-2,4-dihydropyrazol-3-one; 3-Methyl-1-phenyl-2-pyrazoline-5-one, 99%; 4E-901; 5-methyl-2-phenyl-2,4-dihydro-pyrazol-3-one; C13008; D01552; AB00375776_14; AB00375776_15; 2 4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 2,4-dihydro-2-phenyl-5-methyl-3H-pyrazol-3-one; Q335099; Q-200386; SR-01000000135-2; SR-01000000135-3; SR-01000000135-5; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one #; BRD-K35458079-001-04-2; BRD-K35458079-001-12-5; BRD-K35458079-001-23-2; Z50145861; F0391-0021; 3-Methyl-1-phenyl-2-pyrazoline-5-one, SAJ special grade; 3-Methyl-1-phenyl-2-pyrazoline-5-one, purum, >=98.0% (NT); 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (Edaravone); Phenazone impurity A, European Pharmacopoeia (EP) Reference Standard; Antipyrine Related Compound A, United States Pharmacopeia (USP) Reference Standard
|
 click to show the detail info of this DFM
click to show the detail info of this DFM