| General Information of API (ID: D01187) |
| Name |
Ivosidenib
|
| Synonyms |
Click to Show/Hide the Synonyms of This API
Ivosidenib; 1448347-49-6; AG120; AG-120; Tibsovo; UNII-Q2PCN8MAM6; Q2PCN8MAM6; 1448347-49-6 (Ivosidenib); (S)-N-((S)-1-(2-chlorophenyl)-2-((3,3-difluorocyclobutyl)amino)-2-oxoethyl)-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide; (2S)-N-[(1S)-1-(2-chlorophenyl)-2-[(3,3-difluorocyclobutyl)amino]-2-oxoethyl]-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide; RG120; ivosidenibum; (2S)-N-{(1S)-1-(2-chlorophenyl)-2-[(3,3-difluorocyclobutyl)amino]-2-oxoethyl}-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide; 1448346-63-1; Ivosidenib [INN]; Tibsovo (TN); Ivosidenib [USAN]; Ivosidenib [WHO-DD]; Ivosidenib (USAN/INN); Ivosidenib [USAN:INN]; GTPL9217; CHEMBL3989958; SCHEMBL15122512; EX-A992; CHEBI:145430; BDBM363689; AMY38924; US9850277, Compound 176; MFCD29036964; NSC789102; s8206; ZINC205136523; CCG-270141; CS-5122; DB14568; NSC-789102; NCGC00476170-04; NCGC00476170-06; (S)-N-((S)-1-(2-chlorophenyl)-2-(3,3-difluorocyclobutylamino)-2-oxoethyl)-1-(4-cyanopyridin-2-yl)-N-; AC-32624; AS-35058; HY-18767; A14386; D11090; Q27895417; (2S)-1-(4-Cyano-2-pyridinyl)-5-oxo-L-prolyl-2-(2-chlorophenyl)-N-(3,3-difluorocyclobutyl)-N2-(5-fluoro-3-pyridinyl)-glycinamide; (S)-N-((S)-1-(2-chlorophenyl)-2-(3,3-difluorocyclobutylamino)-2-oxoethyl)-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide; Glycinamide, 1-(4-cyano-2-pyridinyl)-5-oxo-L-prolyl-2-(2-chlorophenyl)-N-(3,3-difluorocyclobutyl)-N2-(5-fluoro-3-pyridinyl)-, (2S)-; N-{(1S)-1-(2-chlorophenyl)-2-[(3,3-difluorocyclobutyl)amino]-2-oxoethyl}-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxo-L-prolinamide
|
| Clinical Status |
Approved
|
| PubChem CID |
|
| Formula |
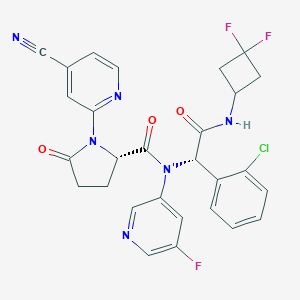
C28H22ClF3N6O3
|
| Canonical SMILES |
C1CC(=O)N([C@@H]1C(=O)N(C2=CC(=CN=C2)F)[C@@H](C3=CC=CC=C3Cl)C(=O)NC4CC(C4)(F)F)C5=NC=CC(=C5)C#N
|
| InChI |
1S/C28H22ClF3N6O3/c29-21-4-2-1-3-20(21)25(26(40)36-18-11-28(31,32)12-18)37(19-10-17(30)14-34-15-19)27(41)22-5-6-24(39)38(22)23-9-16(13-33)7-8-35-23/h1-4,7-10,14-15,18,22,25H,5-6,11-12H2,(H,36,40)/t22-,25-/m0/s1
|
| InChIKey |
WIJZXSAJMHAVGX-DHLKQENFSA-N
|
|
Click to Show/Hide the Molecular Data (Structure/Property) of This API
|
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=71657455"></iframe>
|
 |
|
3D MOL is unavailable
|
2D MOL
|
| Physicochemical Properties |
Molecular Weight |
583 |
Topological Polar Surface Area |
119 |
| XlogP |
3.4 |
Complexity |
1050 |
| Heavy Atom Count |
41 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
9 |
|
|
|
|
|
|
 click to show the detail info of this DFM
click to show the detail info of this DFM

