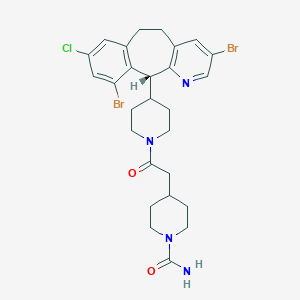
Details of the Active Pharmaceutical Ingredient (API)
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Lonafarnib 50 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Croscarmellose Sodium; Magnesium Stearate; Poloxamer 188; Povidone; Silicon Dioxide; Gelatin; Titanium Dioxide; Yellow Iron Oxide; Red Iron Oxide; Ammonia Solution; Black Iron Oxide; Butyl Alcohol; Dehydrated Alcohol; Isopropyl Alcohol; Potassium Hydroxide; Propylene Glycol; Purified Water; Shellac
|
|||||
| Dosage Form | Capsule | |||||
| Company | Eiger Biopharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [1] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [2] | |||
| Gelatin | DIG Info | Mephenytoin 4-hydroxylase (EC50 = 20.5 uM) | [3] | |||
| Poloxamer 188 | DIG Info | Albendazole monooxygenase (IC50 = 60 uM) | [3] | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Lonafarnib 75 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Croscarmellose Sodium; Magnesium Stearate; Poloxamer 188; Povidone; Silicon Dioxide; Gelatin; Titanium Dioxide; Yellow Iron Oxide; Red Iron Oxide; Ammonia Solution; Black Iron Oxide; Butyl Alcohol; Dehydrated Alcohol; Isopropyl Alcohol; Potassium Hydroxide; Propylene Glycol; Purified Water; Shellac
|
|||||
| Dosage Form | Capsule | |||||
| Company | Eiger Biopharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [1] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [2] | |||
| Gelatin | DIG Info | Mephenytoin 4-hydroxylase (EC50 = 20.5 uM) | [3] | |||
| Poloxamer 188 | DIG Info | Albendazole monooxygenase (IC50 = 60 uM) | [3] | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.