Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00351) | |||||
|---|---|---|---|---|---|
| Name |
Lapatinib
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Lapatinib; 231277-92-2; Tykerb; GW572016; Lapatinib base; GW 572016; Tyverb; 388082-78-8; Lapatinib tosilate hydrate; Lapatinib free base; UNII-0VUA21238F; Lapatinib (free base); 231277-92-2 (free base); MFCD09264194; CHEMBL554; GW-572016; CHEBI:49603; 0VUA21238F; NSC745750; NCGC00167507-01; C29H26ClFN4O4S; DSSTox_CID_26675; DSSTox_RID_81812; DSSTox_GSID_46675; Lapatinib (INN); GSK 572016; CAS-231277-92-2; GW-2016; Lapatinib [INN:BAN]; GSK572016; GW 282974X; HSDB 8209; Lapatinib Ditosylate/; PubChem7650; nchembio866-comp20; Kinome_3684; Kinome_3685; SCHEMBL8100; BDBM5445; cid_208908; GTPL5692; QCR-63; DTXSID7046675; AOB5254; EX-A402; SYN1052; BCPP000188; BCPP000189; HMS2089H10; HMS3244N06; HMS3244N10; HMS3244N14; HMS3744K11; Tykerb (TN) (Glaxo Smith Kline); BCP01874; ZINC1550477; Tox21_112505; ABP000866; ABP000872; NSC800780; AKOS005145766; Tox21_112505_1; AC-1314; BCP9000837; BCP9000838; CCG-270133; CM14421; DB01259; GSK-572016; GW-572016X; NSC-745750; NSC-800780; SB16918; NCGC00167507-02; NCGC00167507-03; NCGC00167507-04; NCGC00167507-09; 913989-15-8; AK-46487; AS-14065; HY-50898; AB0005824; AM20090641; FT-0659650; SW199101-5; A25184; D08108; J90020; W-3749; 49311-EP2298768A1; 49311-EP2298778A1; 49311-EP2311840A1; AB01273965-01; AB01273965-02; AB01273965-03; AB01273965_04; AB01273965_05; 277L922; Q420323; Q-101353; SR-05000001472-1; BRD-K19687926-001-01-7; BRD-K19687926-379-02-5; GW572016;GW-572016;GW 572016; 1092929-10-6
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Breast cancer | ICD-11: 2C60 | [1] | ||
| PubChem CID | |||||
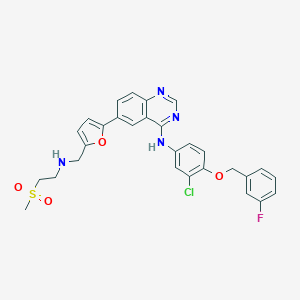
| Formula |
C29H26ClFN4O4S
|
||||
| Canonical SMILES |
CS(=O)(=O)CCNCC1=CC=C(O1)C2=CC3=C(C=C2)N=CN=C3NC4=CC(=C(C=C4)OCC5=CC(=CC=C5)F)Cl
|
||||
| InChI |
1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35)
|
||||
| InChIKey |
BCFGMOOMADDAQU-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=208908"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 581.1 | Topological Polar Surface Area | 115 | |
| XlogP | 5.1 | Complexity | 898 | ||
| Heavy Atom Count | 40 | Rotatable Bond Count | 11 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 9 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Lapatinib 250 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c yellow no. 6; Magnesium stearate; Titanium dioxide; Polysorbate 80; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycols; Povidones; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | GlaxoSmithKline; Novartis Pharmaceuticals Corporation | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Lapatinib Ditosylate eq 250mg base tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Magnesium Stearate; Microcrystalline Cellulose; Povidone; Sodium Starch Glycolate; Fd&C Yellow No. 6/Sunset Yellow Fcf Aluminum Lake; Hypromellose; Macrogol/Peg 400; Polysorbate 80; Titanium Dioxide
|
|||||
| Dosage Form | Tablet | |||||
| Company | Novartis | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Hypromellose | DIG Info | Cytochrome P450 3A5 (IC50 = 19.4 uM) | [5] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [3] | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [5] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Polyethylene glycol 400 | DIG Info | Albendazole monooxygenase (IC50 = 10.77 mg.mL-1) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
