Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00382) | |||||
|---|---|---|---|---|---|
| Name |
Macitentan
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Macitentan; 441798-33-0; Opsumit; ACT-064992; ACT 064992; UNII-Z9K9Y9WMVL; ACT064992; N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide; Z9K9Y9WMVL; CHEBI:76607; Actelion-1; N-[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl]-N'- propylsulfamide; Macitentan [USAN:INN]; macitentanum; zlchem 5; Opsumit (TN); Macitentan (JAN/USAN); (non-labelled)Macitentan-d7; MLS006011174; C19H20Br2N6O4S; GTPL7352; SCHEMBL1445625; CHEMBL2103873; DTXSID50196063; EX-A544; ZLA0005; HMS3653N06; HMS3747E09; cas:441798-33-0;Macitentan; AOB87765; BCP05309; ABP001066; BDBM50395626; MFCD17167076; ZINC43202140; AKOS024463406; ACN-034475; AM81244; CCG-270155; CS-0686; DB08932; SB14841; Macitentan (Actelion-1,ACT-064992); NCGC00346456-01; NCGC00346456-05; 5-(4-bromophenyl)-6-[2-(5-bromopyrimidin-2-yl)oxyethoxy]-N-(propylsulfamoyl)pyrimidin-4-amine; AC-30102; AK162704; AS-74590; HY-14184; QC-10426; SMR004702943; AB0007893; DB-070519; FT-0696675; SW219473-1; A11492; ACT 064992; ACT-064992; D10135; S-7749; Q6724151; {[5-(4-BROMOPHENYL)-6-{2-[(5-BROMOPYRIMIDIN-2-YL)OXY]ETHOXY}PYRIMIDIN-4-YL]SULFAMOYL}(PROPYL)AMINE; N-(5-(4-Bromophenyl)-6-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)pyrimidin-4-yl)-N'-propylsulfamide; N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)-oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide; n-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-n'-propyl-sulfamide; N-[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl]-N'-propylsulfuric diamide; Sulfamide, N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl-
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Pulmonary hypertension | ICD-11: BB01 | [1] | ||
| PubChem CID | |||||
| Formula |
C19H20Br2N6O4S
|
||||
| Canonical SMILES |
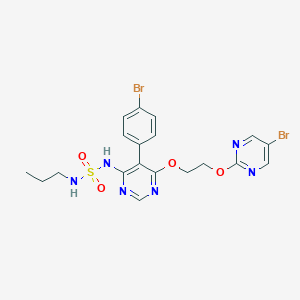
CCCNS(=O)(=O)NC1=C(C(=NC=N1)OCCOC2=NC=C(C=N2)Br)C3=CC=C(C=C3)Br
|
||||
| InChI |
1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27)
|
||||
| InChIKey |
JGCMEBMXRHSZKX-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=16004692"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 588.3 | Topological Polar Surface Area | 137 | |
| XlogP | 3.7 | Complexity | 642 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 11 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 10 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Macitentan 10 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Magnesium stearate; Talc; Titanium dioxide; Lecithin, soybean; Polysorbate 80; Microcrystalline cellulose; Polyvinyl alcohol, unspecified; Povidone, unspecified; Sodium starch glycolate type a potato; Xanthan gum
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Actelion Pharmaceuticals US | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
