Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00429) | |||||
|---|---|---|---|---|---|
| Name |
Miconazole
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
miconazole; 22916-47-8; Monistat; Monistat IV; Daktarin IV; Miconazol; Miconazolo; Miconazolum; Minostate; Monistat-Derm; 1-[2-(2,4-Dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1H-imidazole; MJR 1762; Miconazolum [INN-Latin]; Vusion; Florid(nitrate); 1-(2-((2,4-Dichlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole; Brentan; R 18134; 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]imidazole; Miconazole 3; NSC 170986; C18H14Cl4N2O; Daktarin; Miconazole (Monistat); Miconazole 7; R18134 nitrate; Monistat 3; Monistat 5; Monistat 7; 1H-Imidazole, 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-; Monistat 1 Combination Pack; CHEMBL91; Monistat (TN); MFCD00216019; Miconazole 7 Combination Pack; 1-(2,4-Dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole; 1H-Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-; Dactarin; CHEBI:82892; M-zole 3 Combination Pack; Monistat 3 Combination Pack; 22916-47-8 (free); NSC-170986; Miconazolo [DCIT]; NCGC00016770-01; Micozole; Zimycan; 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl) imidazole; Imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-; Femizol-M; Miconazole-7; Monazole 7; 1-[2-(2,4-dichlorophenyl)-2-{[(2,4-dichlorophenyl)methyl]oxy}ethyl]-1H-imidazole; Miconazol [INN-Spanish]; 75319-47-0; Oravig; Aflorix(nitrate); 1-(2,4-dichlorophenyl)-1-[(2,4-dichlorophenyl)methoxy]-2-imidazolylethane; 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole; 1-{2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole; imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy) phenethyl)-; Albistat(nitrate); Andergin(nitrate); Conofite(nitrate); 1H-imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl) methoxy)ethyl)-; Monista (nitrate); Micantin (nitrate); Novo-Miconazole Vaginal Ovules; CCRIS 7924; Gyno-Daktar(nitrate); Lotrimin AF(nitrate); NSC169434; Epi-Monistat(nitrate); Monistat 7 Vaginal Suppositories; EINECS 245-324-5; BRN 0965511; Zimybase; Miconazole Base; 1-(2,4-Dichloro-beta-[(2,4-dichlorobenzyl)oxy]phenethyl)imidazole; R-14889; SR-01000000271; 1-(2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole; 1-[2-[(2,4-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole; Miconazole [USP:INN:BAN:JAN]; Prestwick_335; Oravig (TN); Spectrum_000965; Prestwick0_000067; Prestwick1_000067; Prestwick2_000067; Prestwick3_000067; Spectrum2_001048; Spectrum3_000507; Spectrum4_000061; Spectrum5_001297; DSSTox_CID_3319; bmse000924; (+-)-1-(2,4-Dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole; cid_4189; SCHEMBL2866; DSSTox_RID_76975; DSSTox_GSID_23319; Oprea1_091955; BSPBio_000253; BSPBio_002033; KBioGR_000581; KBioSS_001445; MLS002222203; DivK1c_000156; SPBio_000976; SPBio_002174; BPBio1_000279; GTPL2449; Miconazole (JP17/USP/INN); DTXSID6023319; SCHEMBL13934598; BDBM31772; KBio1_000156; KBio2_001445; KBio2_004013; KBio2_006581; KBio3_001533; NINDS_000156; HMS1568M15; HMS2090B21; HMS2095M15; HMS2232B14; HMS3374J10; HMS3656E14; HMS3712M15; HY-B0454; Tox21_110601; ANW-42336; DL-448; NSC170986; SBB057453; STK834405; AKOS001574474; AKOS016842489; CCG-220067; DB01110; DS-1881; MCULE-2106181573; IDI1_000156; Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-; NCGC00018294-02; NCGC00018294-04; NCGC00018294-06; NCGC00018294-08; AK-34234; NCI60_001353; NCI60_001380; SMR001307249; ST024747; SBI-0051448.P003; AB0023582; CAS-22916-47-8; DB-046018; AB00053500; FT-0628942; SW196614-4; D00416; J10390; M-4631; AB00053500-23; AB00053500-24; AB00053500-25; AB00053500_26; AB00053500_27; AB00053500_28; 216M019; AE-641/01941016; Q410534; J-014898; SR-01000000271-5; BRD-A82396632-001-03-0; BRD-A82396632-008-02-7; 1-[2,4dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole; 1-[2,4-Dichloro-beta-([2,4-dichlorobenzyl]oxy)-phenethyl]imidazole; 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)-phenethyl]imidazole; 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]-imidazole; 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole; Imidazole,4-dichloro-.beta.-[(2,4-dichlorobenzyl)oxy]phenethyl]-; 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-imidazol; 1-[2,4-Dichloro-.beta.-[(2,4-dichlorobenzyl)oxy]phenethyl]imidazole; 1-[2,4-Dichloro-beta-([2,4-dichlorobenzyl]oxy)-phenethyl] imidazole; 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole; 1H-Imidazole,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-; Imidazole, 1-(2,4-dichloro-.beta.-((2,4-dichlorobenzyl)oxy)phenethyl)-; 1-[2-(2,4-Dichloro-benzyloxy)-2-(2,4-dichloro-phenyl)-ethyl]-1H-imidazole; 1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl)ethyl]imidazole;nitric acid; 1-[2-(2,4-Dichlorophenyl)-2-[(2,4-dichlorophenyl)-methoxy]ethyl]-1H-imidazole; 1-[2-[(2,4-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole #
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Dermatophytosis | ICD-11: 1F28 | [1] | ||
| PubChem CID | |||||
| Formula |
C18H14Cl4N2O
|
||||
| Canonical SMILES |
C1=CC(=C(C=C1Cl)Cl)COC(CN2C=CN=C2)C3=C(C=C(C=C3)Cl)Cl
|
||||
| InChI |
1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2
|
||||
| InChIKey |
BYBLEWFAAKGYCD-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
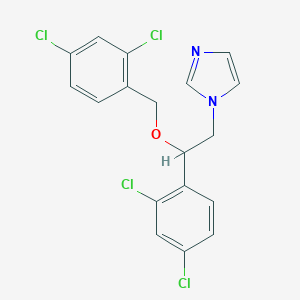
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4189"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 416.1 | Topological Polar Surface Area | 27 | |
| XlogP | 5.3 | Complexity | 417 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 6 | ||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Miconazole 50 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Talc; Casein; Hypromellose, unspecified; Starch, corn
|
|||||
| Dosage Form | Buccal Tablet | |||||
| Company | Midatech Pharma US | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Talc; Casein; Hypromelloses; Starch, corn
|
|||||
| Dosage Form | Buccal Tablet | |||||
| Company | Praelia Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
