Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00432) | |||||
|---|---|---|---|---|---|
| Name |
Mifepristone
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
mifepristone; 84371-65-3; Mifegyne; Mifeprex; RU-486; Corlux; Korlym; RU 486; RU 38486; Mifepriston; RU-38486; UNII-320T6RNW1F; MLS000069785; 320T6RNW1F; VGX-410C; CHEBI:50692; VGX-410; Mifepristone, 98%; NCGC00025179-05; SMR000058481; Mifepristonum [Latin]; Mifepristona [Spanish]; DSSTox_CID_3322; DSSTox_RID_76976; DSSTox_GSID_23322; Mifepristona; Mifepristonum; RU486 (tetramethyl-rhodamine conjugated); Mifepristone [USAN:INN:BAN]; HSDB 6841; SR-01000076011; R 38486; BRN 5779404; Pictovir; RU 486-6; CCRIS 9332; Mifeprex (TN); Pictovir (TM); Prestwick_570; CAS-84371-65-3; Korlym (TN); Mifepristone (Mifeprex); Mifepristone(Mifeprex)/; Opera_ID_562; Mifepristone, >=98%; Prestwick0_000299; Prestwick1_000299; Prestwick2_000299; Prestwick3_000299; Spectrum5_002045; BIDD:PXR0123; Lopac0_000801; SCHEMBL16087; BSPBio_000238; MLS001074115; MLS001424271; (non-labelled)Mifepristone-d3; ARONIS27015; SPBio_002457; RU-486; MIFEPRISTONE; BPBio1_000262; CHEMBL438575; GTPL2805; Mifepristone (JAN/USAN/INN); CHEMBL1276308; DTXSID5023322; BDBM18627; AOB6893; HMS1568L20; HMS2052L05; HMS2090L22; HMS2095L20; HMS2230P21; HMS3262B03; HMS3412D17; HMS3649J08; HMS3676D17; HMS3712L20; HMS3884D12; ACT02598; BCP02145; ZINC3831128; Tox21_110952; Tox21_301841; Tox21_500801; ABP000437; ANW-41472; BDBM50072024; HSCI1_000369; MFCD00867226; VX-410; AKOS015895416; Tox21_110952_1; CCG-101164; CI-1073; CS-1435; DB00834; LP00801; NC00414; SDCCGSBI-0050778.P002; Mifepristone 1.0 mg/ml in Acetonitrile; NCGC00025179-06; NCGC00025179-07; NCGC00025179-08; NCGC00025179-09; NCGC00025179-12; NCGC00025179-13; NCGC00025179-23; NCGC00179632-01; NCGC00255152-01; NCGC00261486-01; AS-13938; CPD000058481; HY-13683; RU486;C-1073; EU-0100801; C07652; D00585; M 8046; W-5163; 29523-EP2272827A1; 29523-EP2275420A1; 29523-EP2295055A2; 29523-EP2295416A2; 29523-EP2298748A2; 29523-EP2298764A1; 29523-EP2298765A1; 29523-EP2305642A2; 29523-EP2308880A1; 29523-EP2311453A1; 29523-EP2311808A1; 29523-EP2311829A1; 29523-EP2311842A2; 75603-EP2269977A2; 75603-EP2308880A1; 371M653; A840767; Q411240; SR-01000721888; Q-201405; SR-01000076011-1; SR-01000076011-3; SR-01000076011-5; SR-01000076011-9; SR-01000721888-4; BRD-K37270826-001-04-5; BRD-K37270826-001-31-8; Mifepristone, United States Pharmacopeia (USP) Reference Standard; 11ss-[p-(Dimethylamino)fenyl]-17ss-hydroxy- 17-(1-propynyl)estra-4,9-dien-3-on; 122742-25-0; 640736-79-4; 83203-42-3
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Cushing syndrome | ICD-11: 5A70 | [1] | ||
| PubChem CID | |||||
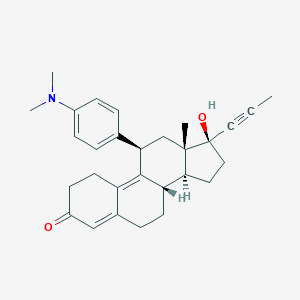
| Formula |
C29H35NO2
|
||||
| Canonical SMILES |
CC#C[C@@]1(CC[C@@H]2[C@@]1(C[C@@H](C3=C4CCC(=O)C=C4CC[C@@H]23)C5=CC=C(C=C5)N(C)C)C)O
|
||||
| InChI |
1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1
|
||||
| InChIKey |
VKHAHZOOUSRJNA-GCNJZUOMSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=55245"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 429.6 | Topological Polar Surface Area | 40.5 | |
| XlogP | 3.8 | Complexity | 921 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 3 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Mifepristone 300 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium lauryl sulfate; D&c yellow no. 10; Fd&c yellow no. 6; Magnesium stearate; Titanium dioxide; Triacetin; Polysorbate 80; Silicon dioxide; Aluminum oxide; Cellulose, microcrystalline; Hydroxypropyl cellulose (type h); Hypromelloses; Sodium starch glycolate type a potato
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Corcept Therapeutics | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [3] | |||
| Polysorbate 80 | DIG Info | Prostaglandin G/H synthase 1 (IC50 = 1 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
