Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00469) | |||||
|---|---|---|---|---|---|
| Name |
Nilutamide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
nilutamide; 63612-50-0; Anandron; Nilandron; 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)imidazolidine-2,4-dione; Nilutamida; Nilutamidum; 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione; RU-23908; RU 23908; 5,5-Dimethyl-3-(alpha,alpha,alpha-trifluoro-4-nitro-m-tolyl)hydantoin; Nilandrone; UNII-51G6I8B902; Nilandron;RU 23908; CHEMBL1274; CHEBI:7573; Nilutamidum [Latin]; Nilutamida [Spanish]; 5,5-Dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)-2,4-imidazolidinedione; 5,5-Dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione; MFCD00864670; 51G6I8B902; NCGC00015754-08; CAS-63612-50-0; 2,4-Imidazolidinedione,5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-; DSSTox_CID_14165; DSSTox_RID_79118; DSSTox_GSID_34165; Nilutamide [USAN:INN:BAN]; 5,5-Dimethyl-3-(4-nitro-3-(trifluoromethyl)-phenyl)imidazolidine-2,4-dione; 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-1,3-diazolidine-2,4-dione; Nilandron (TN); RU 23908-10; SR-01000076034; Nilutamide (USAN/INN); BRN 0841906; Nilutamide, solid; 1-(3'-Trifluoromethyl-4'-nitrophenyl)-4,4-dimethylimidazolidine-2,5-dione; PubChem19359; Spectrum_001625; Tocris-1759; SpecPlus_000902; Prestwick0_000928; Prestwick1_000928; Prestwick2_000928; Prestwick3_000928; Spectrum2_001973; Spectrum3_001633; Spectrum4_000600; Spectrum5_001512; Lopac-N-8534; N 8534; 2,4-IMIDAZOLIDINEDIONE, 5,5-DIMETHYL-3-(4-NITRO-3-(TRIFLUOROMETHYL)PHENYL)-; BIDD:PXR0177; Lopac0_000879; SCHEMBL12670; BSPBio_000836; BSPBio_003325; KBioGR_001100; KBioSS_002105; MLS002154066; BIDD:GT0683; DivK1c_006998; SPECTRUM1504152; SPBio_002125; SPBio_003015; BPBio1_000920; GTPL2864; DTXSID3034165; KBio1_001942; KBio2_002105; KBio2_004673; KBio2_007241; KBio3_002545; BCPP000148; HMS1570J18; HMS1922F03; HMS2093A10; HMS2097J18; HMS2230E03; HMS3262P19; HMS3268C18; HMS3369I02; HMS3414N15; HMS3678N13; HMS3714J18; Pharmakon1600-01504152; BCP26617; ZINC3874498; Tox21 110213; Tox21_110213; Tox21_301589; Tox21_500879; ANW-58327; BDBM50135912; CCG-39427; NSC758683; SBB067297; STK633161; AKOS005565152; AKOS025147305; Tox21_110213_1; AC-5260; BCP9000990; DB00665; LP00879; MCULE-3215206804; NSC-758683; SB19036; SDCCGSBI-0050854.P004; NCGC00015754-01; NCGC00015754-02; NCGC00015754-03; NCGC00015754-04; NCGC00015754-05; NCGC00015754-06; NCGC00015754-07; NCGC00015754-09; NCGC00015754-10; NCGC00015754-11; NCGC00015754-12; NCGC00015754-15; NCGC00015754-16; NCGC00015754-22; NCGC00025280-01; NCGC00025280-02; NCGC00025280-03; NCGC00025280-04; NCGC00025280-05; NCGC00025280-06; NCGC00025280-07; NCGC00025280-08; NCGC00255271-01; NCGC00261564-01; AK-82300; AS-14123; HY-13702; SMR001233381; ST075550; SBI-0050854.P003; AB0013969; AB00053180; CS-0007719; EU-0100879; FT-0630740; C08164; D00965; AB00053180_07; 612N500; A834440; L000759; Q3877030; RU-23908;RU 23908;RU23908; SR-01000076034-1; SR-01000076034-3; SR-01000076034-5; SR-01000076034-6; SR-01000076034-9; BRD-K23566484-001-05-2; BRD-K23566484-001-09-4; Z2417927201; Nilutamide, European Pharmacopoeia (EP) Reference Standard; 1-(3'-trifluoromethyl-4'-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione; 1-(3'-trifluoromethyl-4'-nitrophenyl)4,4-dimethyl-imidazoline-2,5-dione; 1-(3'-trifluoromethyl-4'nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione; 1-(3'trifluoromethyl-4'-nitropheyl)-4,4-dimethyl-imidazoline-2,5-dione; 1-(3-'trifluoromethyl-4'-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione; 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl imidazolidine-2,5-dione; 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl-imidazolidine-2,5-dione; 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl-imidazoline-2,5-dione; 1-(3-trifluoromethyl-4-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione; 3-(3-(trifluoromethyl)-4-nitrophenyl)-5,5-dimethylimidazolidine-2,4-dione; 5,5-Dimethyl-3-(4-nitro-3-trifluoromethyl-phenyl)-imidazolidine-2,4-dione; diethyl1,4-dihydro-2,6-dimethyl-1,4-diphenyl-3,5-pyridinedicarboxylate; 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-imidazolidine-2,4-dione
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Prostate cancer | ICD-11: 2C82 | [1] | ||
| PubChem CID | |||||
| Formula |
C12H10F3N3O4
|
||||
| Canonical SMILES |
CC1(C(=O)N(C(=O)N1)C2=CC(=C(C=C2)[N+](=O)[O-])C(F)(F)F)C
|
||||
| InChI |
1S/C12H10F3N3O4/c1-11(2)9(19)17(10(20)16-11)6-3-4-8(18(21)22)7(5-6)12(13,14)15/h3-5H,1-2H3,(H,16,20)
|
||||
| InChIKey |
XWXYUMMDTVBTOU-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4493"></iframe>
|
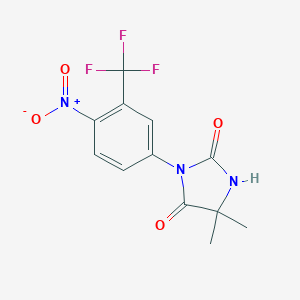 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 317.22 | Topological Polar Surface Area | 95.2 | |
| XlogP | 2 | Complexity | 515 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 1 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Nilutamide 150 mg tablet | Click to Show/Hide the Full List of Formulation(s): 3 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Docusate sodium; Magnesium stearate; Talc; Povidone; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Sanofi-Aventis | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Docusate sodium | DIG Info | Solute carrier SLCO2B1 (Ki = 2.3 uM) | [2] | |||
| Povidone | DIG Info | Cholesterol 25-hydroxylase (IC50 = 78.3 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose; Docusate sodium; Magnesium stearate; Talc; Povidones; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Concordia Pharmaceuticals; Covis Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Docusate sodium | DIG Info | Solute carrier SLCO2B1 (Ki = 2.3 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Docusate sodium; Calcium stearate; Talc; Povidone k30; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | ANI Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Docusate sodium | DIG Info | Solute carrier SLCO2B1 (Ki = 2.3 uM) | [2] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
