Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00535) | |||||
|---|---|---|---|---|---|
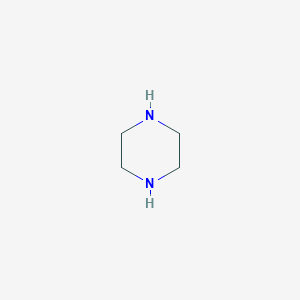
| Name |
Piperazine
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
piperazine; 110-85-0; Diethylenediamine; 1,4-Diazacyclohexane; Piperazin; Hexahydropyrazine; Piperazidine; Antiren; 1,4-Piperazine; Diethyleneimine; Eraverm; Pipersol; Dispermine; Lumbrical; Wurmirazin; Uvilon; Piperazine, anhydrous; Vermex; 1,4-Diethylenediamine; Worm-A-Ton; Asca-Trol No. 3; Pyrazine hexahydride; Hexahydro-1,4-diazine; Pyrazine, hexahydro-; Vermizine (TN); Piperazine (USP); NSC 474; UNII-1RTM4PAL0V; MFCD00005953; Piperazine [USP]; 1RTM4PAL0V; NSC474; CHEBI:28568; Eraverm (VAN); Piperazin [German]; NCGC00094762-03; Upixon; DSSTox_CID_1164; DSSTox_RID_75986; DSSTox_GSID_21164; Piperazine anhydrous; Piperazine, 99%, extra pure; Piperazin [Germany]; Anhydrous piperazine; CAS-110-85-0; CCRIS 5950; HSDB 1093; EINECS 203-808-3; UN2579; BRN 0102555; piperizine; piperzine; piprazine; exahydropyrazine; Piperazine-; 7-piperazine; 4-diazacyclohexane; Piperazine, 99%; Tasnon (Salt/Mix); Exelmin (Salt/Mix); Vermidol (Salt/Mix); Vermizine (Salt/Mix); PubChem19047; Spectrum_001113; ACMC-1AWXD; Spectrum5_001817; WLN: T6M DMTJ; EC 203-808-3; Piperazine, p.a., 98%; CHEMBL1412; NCIOpen2_000984; NCIOpen2_000988; NCIOpen2_001024; NCIOpen2_001031; NCIOpen2_001033; NCIOpen2_001071; NCIOpen2_001073; NCIOpen2_001111; NCIOpen2_001151; NCIOpen2_001231; NCIOpen2_001262; NCIOpen2_001269; NCIOpen2_004830; NCIOpen2_004834; NCIOpen2_004862; NCIOpen2_004874; NCIOpen2_004904; NCIOpen2_004910; NCIOpen2_004914; NCIOpen2_004942; NCIOpen2_004952; NCIOpen2_004954; NCIOpen2_004982; NCIOpen2_004992; NCIOpen2_004994; NCIOpen2_005022; NCIOpen2_005032; NCIOpen2_005034; NCIOpen2_005062; NCIOpen2_005072; NCIOpen2_005102; NCIOpen2_005108; NCIOpen2_005145; NCIOpen2_005182; NCIOpen2_005185; NCIOpen2_005187; NCIOpen2_005200; NCIOpen2_005575; NCIOpen2_005980; NCIOpen2_009422; KBioSS_001593; 5-23-01-00030 (Beilstein Handbook Reference); ARONIS23910; BIDD:GT0273; DivK1c_000038; SPECTRUM1500490; Piperazine, analytical standard; DTXSID1021164; HMS500B20; KBio1_000038; KBio2_001593; KBio2_004161; KBio2_006729; NSC-474; MANGANESE(II)SILICOFLUORIDE; NINDS_000038; HMS1920H20; HMS2092A03; HMS3885L08; Pharmakon1600-01500490; 1$l^{2},4$l^{2}-diazinane; ACT02177; BCP24060; HY-B0912; Piperazine, ReagentPlus(R), 99%; STR00051; ZINC5850277; Tox21_113564; Tox21_202242; Tox21_300104; ANW-16201; BBL019924; NSC757283; STL169348; Piperazine [UN2579] [Corrosive]; AKOS000269028; CCG-212753; CS-4381; DB00592; LF-0561; MCULE-5522245554; NSC-757283; UN 2579; IDI1_000038; NCGC00094762-01; NCGC00094762-02; NCGC00094762-04; NCGC00094762-05; NCGC00094762-08; NCGC00254077-01; NCGC00259791-01; Piperazine, anhydrous, >=99.0% (T); 8057-14-5; SBI-0051485.P003; FT-0673919; AZ0001-0086; 5726-EP2269610A2; 5726-EP2269977A2; 5726-EP2269993A1; 5726-EP2270002A1; 5726-EP2270006A1; 5726-EP2270010A1; 5726-EP2272509A1; 5726-EP2272517A1; 5726-EP2272813A2; 5726-EP2272841A1; 5726-EP2272935A1; 5726-EP2272972A1; 5726-EP2272973A1; 5726-EP2275395A2; 5726-EP2275401A1; 5726-EP2275404A1; 5726-EP2275411A2; 5726-EP2275412A1; 5726-EP2275420A1; 5726-EP2275421A1; 5726-EP2277848A1; 5726-EP2277858A1; 5726-EP2277867A2; 5726-EP2277872A1; 5726-EP2277876A1; 5726-EP2277877A1; 5726-EP2277880A1; 5726-EP2280003A2; 5726-EP2280005A1; 5726-EP2280006A1; 5726-EP2280008A2; 5726-EP2280009A1; 5726-EP2280010A2; 5726-EP2281563A1; 5726-EP2281810A1; 5726-EP2281813A1; 5726-EP2281815A1; 5726-EP2281818A1; 5726-EP2281819A1; 5726-EP2281821A1; 5726-EP2284149A1; 5726-EP2284157A1; 5726-EP2284159A1; 5726-EP2284160A1; 5726-EP2287163A1; 5726-EP2289510A1; 5726-EP2289868A1; 5726-EP2289883A1; 5726-EP2289884A1; 5726-EP2289887A2; 5726-EP2289888A2; 5726-EP2289894A2; 5726-EP2292586A2; 5726-EP2292593A2; 5726-EP2292606A1; 5726-EP2292610A1; 5726-EP2292611A1; 5726-EP2292614A1; 5726-EP2292619A1; 5726-EP2292624A1; 5726-EP2292630A1; 5726-EP2295399A2; 5726-EP2295402A2; 5726-EP2295407A1; 5726-EP2295409A1; 5726-EP2295426A1; 5726-EP2295427A1; 5726-EP2295429A1; 5726-EP2295432A1; 5726-EP2295433A2; 5726-EP2295437A1; 5726-EP2295438A1; 5726-EP2298731A1; 5726-EP2298734A2; 5726-EP2298736A1; 5726-EP2298738A1; 5726-EP2298743A1; 5726-EP2298755A1; 5726-EP2298763A1; 5726-EP2298766A1; 5726-EP2298767A1; 5726-EP2298772A1; 5726-EP2298775A1; 5726-EP2298778A1; 5726-EP2298783A1; 5726-EP2300432A1; 5726-EP2301928A1; 5726-EP2301936A1; 5726-EP2301937A1; 5726-EP2305219A1; 5726-EP2305250A1; 5726-EP2305646A1; 5726-EP2305648A1; 5726-EP2305651A1; 5726-EP2305657A2; 5726-EP2305659A1; 5726-EP2305672A1; 5726-EP2305677A1; 5726-EP2305678A1; 5726-EP2305679A1; 5726-EP2305682A1; 5726-EP2305684A1; 5726-EP2305687A1; 5726-EP2308510A1; 5726-EP2308562A2; 5726-EP2308812A2; 5726-EP2308838A1; 5726-EP2308839A1; 5726-EP2308840A1; 5726-EP2308848A1; 5726-EP2308854A1; 5726-EP2308857A1; 5726-EP2308867A2; 5726-EP2308870A2; 5726-EP2308872A1; 5726-EP2308873A1; 5726-EP2308875A1; 5726-EP2308879A1; 5726-EP2309584A1; 5726-EP2311464A1; 5726-EP2311808A1; 5726-EP2311814A1; 5726-EP2311818A1; 5726-EP2311824A1; 5726-EP2311828A1; 5726-EP2311829A1; 5726-EP2311831A1; 5726-EP2311842A2; 5726-EP2314295A1; 5726-EP2314574A1; 5726-EP2314575A1; 5726-EP2314584A1; 5726-EP2314586A1; 5726-EP2314587A1; 5726-EP2314588A1; 5726-EP2314590A1; 5726-EP2315760A1; 5726-EP2316457A1; 5726-EP2316458A1; 5726-EP2316470A2; 5726-EP2316825A1; 5726-EP2316826A1; 5726-EP2316827A1; 5726-EP2316828A1; 5726-EP2316829A1; 5726-EP2316831A1; 5726-EP2316832A1; 5726-EP2316833A1; 5726-EP2316836A1; 5726-EP2371811A2; 5726-EP2371831A1; 5726-EP2372804A1; 5726-EP2374895A1; 5726-EP2378585A1; B-6439; C07973; D00807; 10847-EP2284160A1; 10847-EP2284166A1; 10847-EP2292589A1; 10847-EP2295439A1; 10847-EP2301928A1; 10847-EP2305651A1; 10847-EP2308852A1; 10847-EP2308854A1; 10847-EP2316827A1; 10847-EP2316828A1; AB00052073_03; 164557-EP2281899A2; 164557-EP2287167A1; Piperazine, BioUltra, anhydrous, >=99.0% (T); Q409292; SR-05000001700; Q-201586; SR-05000001700-1; F0001-0226; Z1245537944; Piperazine, United States Pharmacopeia (USP) Reference Standard; 1323940-30-2
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Ascariasis | ICD-11: 1F62 | [1] | ||
| PubChem CID | |||||
| Formula |
C4H10N2
|
||||
| Canonical SMILES |
C1CNCCN1
|
||||
| InChI |
1S/C4H10N2/c1-2-6-4-3-5-1/h5-6H,1-4H2
|
||||
| InChIKey |
GLUUGHFHXGJENI-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4837"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 86.14 | Topological Polar Surface Area | 24.1 | |
| XlogP | -1.5 | Complexity | 26.5 | ||
| Heavy Atom Count | 6 | Rotatable Bond Count | 0 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Piperazine 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c blue no. 1; Isopropyl alcohol; Magnesium stearate; Dibasic calcium phosphate dihydrate; Water; Povidone k30; Cellulose, microcrystalline
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | United Pet Group | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [2] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
