| Synonyms |
Click to Show/Hide the Synonyms of This API
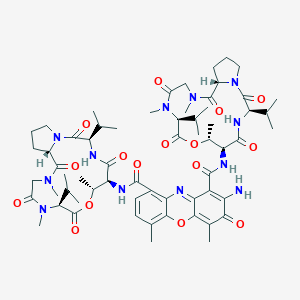
DACTINOMYCIN; actinomycin D; Actinomycin C1; Cosmegen; Actinomycin IV; 50-76-0; Meractinomycin; ActD; ACT D; Dactinomycine [INN-French]; Dactinomycinum [INN-Latin]; UNII-1CC1JFE158; Dactinomicina [INN-Spanish]; Lyovac cosmegen; Dactinomycin D; Oncostatin K; Chounghwamycin B; Actinomycin 7; NCI-C04682; MFCD00005033; Actinomycin Aiv; Actinomycin I; GNF-Pf-2290; MLS001424196; 1CC1JFE158; Actinomycin I1; CHEBI:27666; Actinomycin X 1; Actinomycin A IV; Acto-D; Dilactone actinomycin D acid; Actactinomycin A IV; Actinomycin C(sub1); Dilactone actinomycindioic D acid; NCGC00161622-02; Actinomycin I(sub 1); SMR000469227; DSSTox_CID_31; Actinomycin 11 cosmegen; Cosmegen Lyovac; Lyovac-Cosmegen; CCRIS 9; DSSTox_RID_75330; DSSTox_GSID_20031; 2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis[(3R,6S,7R,10S,16S)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propan-2-yl)-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]phenoxazine-1,9-dicarboxamide; HSDB 3220; Dactinomicina; Dactinomycine; Dactinomycinum; Actinomycindioic D acid, dilactone; Antibiotic from Streptomyces parvullus; Actinomycin-(threo-val-pro-sar-meval); ACTINOMYCIN-D; CAS-50-76-0; ACT [antibiotic]; X 97; 2-amino-N,N'-bis(hexadecahydro-2,5,9-trimethyl-6,13-bis(1-methylethyl)-1,4,7,11,14-pentaoxo-1H-pyrrolo(2,1-i)(1,4,7,10,13)oxatetra-azacyclohexadecin-10-yl)-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide; 2-amino-N1,N9-bis((6S,9R,10S,13R,18aS)-6,13-diisopropyl-2,5,9-trimethyl-1,4,7,11,14-pentaoxohexadecahydro-1H-pyrrolo[2,1-i][1]oxa[4,7,10,13]tetraazacyclohexadecin-10-yl)-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide; AD (VAN); GNF-PF-1977; NSC3053; NSC-3053; Glycopeptide, 4a; 2-amino-4,6-dimethyl-3-oxo-N,N'-bis[(6S,9R,10S,13R,18aS)-2,5,9-trimethyl-6,13-bis(1-methylethyl)-1,4,7,11,14-pentaoxohexadecahydro-1H-pyrrolo[2,1-i][1,4,7,10,13]oxatetraazacyclohexadecin-10-yl]-3H-phenoxazine-1,9-dicarboxamide; NSC 3053; Dactinomycin [USAN:USP:INN:BAN]; EINECS 200-063-6; AI3-26374; UPCMLD-DP055; SCHEMBL3844; CHEMBL1554; cid_457193; DTXSID9020031; UPCMLD-DP055:001; UPCMLD-DP055:002; BDBM43866; HMS2052O17; Tox21_111997; Tox21_202482; BDBM50089528; s8964; AKOS030228553; Tox21_111997_1; CCG-101134; DB00970; NC00384; NCGC00090796-01; NCGC00161622-01; NCGC00260031-01; NCGC00271789-02; 3H-Phenoxazine-1,9-dicarboxamide, 2-amino-N,N'-bis(hexadecahydro-2,5,9-trimethyl-6,13-bis(1-methylethyl)-1,4,7,11,14-pentaoxo-1H-pyrrolo(2,1-i)(1,4,7,10,13)oxatetra-azacyclohexadecin-10-yl)-4,6-dimethyl-3-oxo-; BP-25384; Specific stereoisomer of N,N'-((2-amino-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-diyl)-bis(carbonylimino(2-hydroxypropylidene)carbonyliminoisobutylidenecarbonyl-1,2-pyrrolidinediylcarbonyl(methylimino)methylenecarbonyl))bis(N-methyl-L-valine) dilactone; Stereoisomer of N,N'-((2-amino-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-diyl)bis(carbonylimino(2-(1-hydroxyethyl)-1-oxo-2,1-ethanediyl)imino(2-(1-methylethyl)-1-oxo-2,1-ethanediyl)-1,2-pyrrolidinediylcarbonyl(methylimino) (1-oxo-2,1-ethanediyl)))bis(N-methyl-L-valine)di-xi-lactone; 15160-EP2272827A1; 15160-EP2275420A1; 15160-EP2280012A2; 15160-EP2281815A1; 15160-EP2289892A1; 15160-EP2292615A1; 15160-EP2295055A2; 15160-EP2295416A2; 15160-EP2295426A1; 15160-EP2295427A1; 15160-EP2298748A2; 15160-EP2298764A1; 15160-EP2298765A1; 15160-EP2301928A1; 15160-EP2301933A1; 15160-EP2305640A2; 15160-EP2305642A2; 15160-EP2305671A1; 15160-EP2308833A2; 15160-EP2311453A1; 15160-EP2311808A1; 15160-EP2311827A1; 15160-EP2311829A1; 15160-EP2311842A2; 15160-EP2316832A1; 15160-EP2316833A1; AB00514445-05; 050A760; Q186127; SR-01000763161; SR-01000763161-4; Actinomycin D, from Streptomyces sp., >=95% (HPLC); Actinomycin D, from Streptomyces sp., ~98% (HPLC); BRD-K70578146-001-01-8; BRD-K70578146-001-04-2; UNII-0OCC969V50 component RJURFGZVJUQBHK-IIXSONLDSA-N; Dactinomycin, United States Pharmacopeia (USP) Reference Standard; Actinomycin D, for fluorescence, >=90% (HPLC), from Streptomyces sp.; Actinomycin D, from Streptomyces sp., suitable for cell culture, >=95%; 1-N,9-N-bis[(6S,9R,10S,13R,18aS)-2,5,9-trimethyl-1,4,7,11,14-pentaoxo-6,13-bis(propan-2-yl)-hexadecahydro-1H-pyrrolo[2,1-i]1-oxa-4,7,10,13-tetraazacyclohexadecan-10-yl]-2-amino-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide; 2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis-[(18aS)-10c,14,17-trimethyl-5,8,12,15,18-pentaoxo-6c,13t-di(propan-2-yl)-18ar-hexadecahydro-1H-pyrrolo[2,1-i][1,4,7,10,13]oxatetraazacyclohexadecin-9c-yl]-3H-phenoxazine-1,9-dicarboxamide; 2-amino-4,6-dimethyl-3-oxo-N1,N9-bis[(3R,6S,7R,10S,16S)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propan-2-yl)-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]phenoxazine-1,9-dicarboxamide; 2-amino-N,N''''''''-bis[(3R,6S,7R,10S,16S)-3,10-diisopropyl-2,5,9,12,15-pentaketo-7,11,14-trimethyl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-3-keto-4,6-dimethyl-phenoxazine-1,9-dicarboxamide; 2-amino-N,N''''-bis[(3R,6S,7R,10S,16S)-3,10-diisopropyl-2,5,9,12,15-pentaketo-7,11,14-trimethyl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-3-keto-4,6-dimethyl-phenoxazine-1,9-dicarboxamide; 2-amino-N,N''-bis[(3R,6S,7R,10S,16S)-3,10-diisopropyl-2,5,9,12,15-pentaketo-7,11,14-trimethyl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-3-keto-4,6-dimethyl-phenoxazine-1,9-dicarboxamide; 2-amino-N1,N9-bis[(3R,6S,7R,10S,16S)-3,10-diisopropyl-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-phenoxazine-1,9-dicarboxamide; 2-azanyl-4,6-dimethyl-3-oxidanylidene-N1,N9-bis[(3R,6S,7R,10S,16S)-7,11,14-trimethyl-2,5,9,12,15-pentakis(oxidanylidene)-3,10-di(propan-2-yl)-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]phenoxazine-1,9-dicarboxamide
|
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM