| Synonyms |
Click to Show/Hide the Synonyms of This DIG
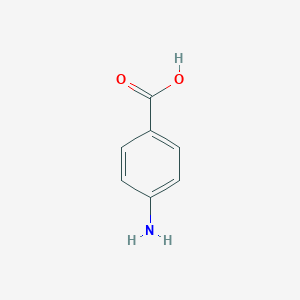
4-aminobenzoic acid; 150-13-0; p-aminobenzoic acid; PABA; para-aminobenzoic acid; AMINOBENZOIC ACID; Vitamin BX; p-Carboxyaniline; 4-Carboxyaniline; Sunbrella; Benzoic acid, 4-amino-; p-Carboxyphenylamine; Hachemina; Paraminol; 1-Amino-4-carboxybenzene; Pabacyd; Pabafilm; Pabamine; Paranate; Amben; Potaba; Romavit; Bacterial vitamin H1; Vitamin H'; Anticanitic vitamin; Chromotrichia factor; RVPaba Lipstick; Papacidum; Rvpaba; Trichochromogenic factor; Anti-chromotrichia factor; Super Shade by Coppertone; Benzoic acid, p-amino-; Acidum paraminobenzoicum; ABEE; Pabagel; Pabanol; 4-AMINO-BENZOIC ACID; Caswell No. 033B; Kyselina p-aminobenzoova; MFCD00007894; Vitamin H1; p-Aminobenzoesaeure; 4-Aminobenzoesaeure; 4-aminobenzoic-acid; 4-Carboxyphenylamine; Acido p-aminobenzoico; PAB; gamma-Aminobenzoic acid; benzoic acid, 4-amino; Acido p-aminobenzoico [Italian]; UNII-TL2TJE8QTX; Kyselina p-aminobenzoova [Czech]; para-aminobenzoate; Anticantic vitamin; EPA Pesticide Chemical Code 233300; BRN 0471605; p-amino benzoic acid; p-amino-Benzoic acid; CHEBI:30753; AI3-02436; TL2TJE8QTX; Aminobenzoic acid (USP); Aminobenzoic acid [USP]; .gamma.-Aminobenzoic acid; Aniline-4-carboxylic acid; CHEMBL542; 4-Aminobenzoic acid, 99%; H-4-ABZ-OH; AMINOBENZOIC ACID, PARA; NCGC00091051-01; 4-aminobenzoicacid; DSSTox_CID_4466; DSSTox_RID_77411; DSSTox_GSID_24466; CAS-150-13-0; SMR000471833; CCRIS 6209; HSDB 6840; NSC 7627; EINECS 205-753-0; Actipol; p-carboxy aniline; p-aminobezoic acid; gamma-Aminobenzoate; 4-aminobezoic acid; Trochromogenic factor; Aniline-4-carboxylate; 161406-19-5; 4-Amino benzoic acid; 4-(amino)benzoic acid; Antichromotrichia factor; PubChem13422; Spectrum_000036; RVPaba lipstick (TN); P-AMINO-BENZOATE; Aminobenzoic Acid, USP; Spectrum2_000123; Spectrum3_000294; Spectrum4_000142; Spectrum5_000778; Tetracaine EP Impurity A; P-Aminobenzoic Acid,(S); ACMC-209d3t; bmse000066; bmse000887; bmse000916; Epitope ID:115017; H-(4)Abz-OH; AMINOBENZOIC-4 ACID; EC 205-753-0; SCHEMBL8249; Oprea1_221096; BSPBio_001828; KBioGR_000584; KBioSS_000396; ZINC920; MLS001066325; MLS001335919; MLS001335920; Para Amino Benzoic Acid USP; BIDD:ER0375; DivK1c_000783; SPBio_000166; PARA AMINO BENZOIC ACID; 4-Aminobenzoic acid-[13C6]; RARECHEM AL BO 0238; component of Presun (Salt/Mix); DTXSID6024466; SCHEMBL13232108; KBio1_000783; KBio2_000396; KBio2_002964; KBio2_005532; KBio3_001328; NSC7627; NINDS_000783; OTAVA-BB 1509951; P-AMINOBENZOIC ACID 100G; 150-13-0 4-aminobenzoic acid; HMS2269E10; HMS3870E13; LABOTEST-BB LTBB000459; WT266; ACT09225; HY-B1008; NSC-7627; Tox21_111069; Tox21_201702; Tox21_300087; ANW-21255; BDBM50145829; LABOTEST-BB LT03328355; s4510; SBB058665; STK397441; AKOS000118983; Tox21_111069_1; AC-8107; AM86626; AS00762; CCG-266139; CS-4505; DB02362; MCULE-3535374816; IDI1_000783; 4-Aminobenzoic acid, analytical standard; NCGC00091051-02; NCGC00091051-03; NCGC00091051-04; NCGC00253923-01; NCGC00259251-01; AK-41178; AS-12493; NCI60_041683; SY003749; SBI-0051277.P003; DB-028695; 4-Aminobenzoic acid, ReagentPlus(R), 99%; A0269; FT-0617557; ST50632536; 4-Aminobenzoic acid, >=98.0% (HPLC/NT); 3297-EP2308861A1; 3297-EP2308868A1; 3297-EP2311809A1; 4-Aminobenzoic acid, ReagentPlus(R), >=99%; 6761-EP1441224A2; 6761-EP2295402A2; C00568; D02456; Q-9193; 48316-EP2281563A1; 48316-EP2308874A1; 48316-EP2311494A1; Q284959; 4-Aminobenzoic acid, Vetec(TM) reagent grade, 98%; Q-200432; 4-Aminobenzoic acid, purified by sublimation, >=99%; Z57127446; 4-Aminobenzoic acid, 98.5-100.2%, SAJ first grade; 4-Aminobenzoic acid, SAJ special grade, 99.5-100.2%; F2191-0259; 4A5E7DD8-8A22-4642-86BF-05B778C0C78E; 4-Aminobenzoic acid, European Pharmacopoeia (EP) Reference Standard; Aminobenzoic acid, United States Pharmacopeia (USP) Reference Standard; Aminobenzoic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
|
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT