| Synonyms |
Click to Show/Hide the Synonyms of This DIG
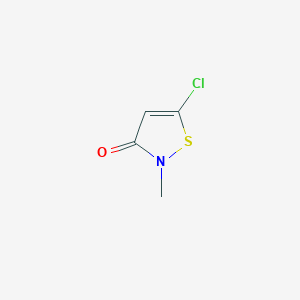
5-Chloro-2-methyl-4-isothiazolin-3-one; 26172-55-4; Methylchloroisothiazolinone; CMIT; 5-Chloro-2-methyl-3(2H)-isothiazolone; 3(2H)-Isothiazolone, 5-chloro-2-methyl-; 5-chloro-2-methylisothiazol-3(2h)-one; 5-Chloro-2-methyl-2H-isothiazol-3-one; 5-chloro-2-methylisothiazolin-3-one; UNII-DEL7T5QRPN; 5-chloro-2-methyl-1,2-thiazol-3-one; Chloromethylisothiazolinone; 5-Chloro-2-methyl-3-isothiazolone; 5-chloro-2-methyl-4-isothiazoline-3-one; DEL7T5QRPN; 5-chloro-n-methylisothiazolin-3-one; 4-ISOTHIAZOLIN-3-ONE, 5-CHLORO-2-METHYL-; 5-chloro-2-methyl-1,2-thiazol-3(2H)-one; CHEBI:53621; 2,3-Dihydro-2-methyl-3-oxo-5-chloroisothiazole; 5-Chloro-2-methyl-4-isothiazolin-3-one (CMI); Kathon CG 5243; NCGC00181041-01; Bioace; Kathon IXE; DSSTox_CID_14286; DSSTox_RID_79138; DSSTox_GSID_34286; CAS-26172-55-4; A 33 (bactericide); HS 818 (antiseptic); EINECS 247-500-7; HS 818; BRN 1210149; n-methyl-5-chloroisothiazolone; N-Methyl-5-chloroisothiazolin-3-one; 1329611-34-8; 5-chloro-2-methyl-2h-isothiazolin-3-one; 5-chloro-2-methyl-3(2H)-isothiazolinone; T 360; SCHEMBL20686; SCHEMBL111860; CHEMBL1738962; DTXSID9034286; HSDB 8270; EBD15202; ZINC2002061; Tox21_112689; Tox21_300199; ANW-44440; MFCD00792550; AKOS006230760; CS-W022348; DB14197; GS-3223; NCGC00181041-02; NCGC00254127-01; 5-Chloro-2-methyl-3-isothiazolone-[d3]; 55965-84-9 CMIT, MIT; AM806586; 2682-20-4 MIT; 5-Chloro-2-methyl-isothiazol-3(2H)-one; AB0011549; DB-007017; FT-0620267; X5928; Q-9256; Q204121; SR-01000944864; 5-chloro-2-methyl-2,3-dihydro-1,2-thiazol-3-one; SR-01000944864-1; W-107193; UNII-15O9QS218W component DHNRXBZYEKSXIM-UHFFFAOYSA-N; 5-Chloro-2-methyl-4-isothiazolin-3-one (>14% solution in water); 5-Chloro-2-methyl-4-isothiazolin-3-one 100 microg/mL in Acetonitrile; N-Methyl-5-chloroisothiazolone (5-Chloro-2-methyl-4-isothiazolin-3-one); 5-Chloro-2-methyl-3(2H)-isothiazolone; 5-Chloro-2-methyl-Isothiazolone; Kathon WT; MCI/MI; 5-Chloro-2-methyl-4-isothiazolin-3-one, tech grade, >14% in water. CMI/MI >2.0; 5-CHLORO-2-METHYL-4-ISOTHIAZOLIN-3-ONE (ACTIVE INGREDIENT >14%, CMI/MI 2.5 - 4.0)
|
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT