Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00473) | |||||
|---|---|---|---|---|---|
| Name |
Nitazoxanide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
NITAZOXANIDE; 55981-09-4; Alinia; Nitazoxamide; 2-((5-nitrothiazol-2-yl)carbamoyl)phenyl acetate; Nitazoxanidum [INN-Latin]; Nitazoxanida [INN-Spanish]; Benzamide, 2-(acetyloxy)-N-(5-nitro-2-thiazolyl)-; 2-[(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl acetate; 2-(Acetolyloxy)-N-(5-nitro-2-thiazolyl)benzamide; C12H9N3O5S; [2-[(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl] acetate; UNII-SOA12P041N; NSC697855; 2-Acetyloxy-N-[(5-nitro-2-thiazolyl)]benzamide; Nitazoxanide (Alinia, Annita); SOA12P041N; Nitazoxanida; 2-(Acetyloxy)-N-(5-nitro-2-thiazolyl)benzamide; MFCD00416599; NSC-697855; PH 5776; PH-5776; NCGC00090774-01; Nitazoxanid; Nitazoxanidum; Colufase; Cryptaz; Heliton; DSSTox_CID_13757; DSSTox_RID_79095; DSSTox_GSID_33757; Phavic-1; acetic acid [2-[[(5-nitro-2-thiazolyl)amino]-oxomethyl]phenyl] ester; SMR000466367; NSC 697855; Alinia (TN); AZT + Nitazoxanide; CAS-55981-09-4; EINECS 259-931-8; Nitazoxanide (USAN/INN); (2-((5-Nitro-2-thiazolyl)carbamoyl)phenyl)acetat; BRN 1225475; N-(5-Nitro-2-thiazolyl)salicylamide acetate (ester); Nitrazoxanide; Pacovanton; Dexidex; Kidonax; Nitazox; Paramix; Nitazoxanide [USAN:INN:BAN]; Alinia(TM); N-(5-Nitrothiazol-2-yl)salicylamide acetate ester; o-(N-(5-Nitrothiazol-2-yl)carbamoyl)phenyl acetate; Salicylamide, N-(5-nitro-2-thiazolyl)-, acetate (ester); CPD000466367; NCIMech_000843; CHEMBL1401; Oprea1_263587; SCHEMBL40981; MLS000759492; MLS001424074; MLS006010127; [2-[(5-nitrothiazol-2-yl)carbamoyl]phenyl] acetate; DTXSID5033757; CHEBI:94807; Nitazoxanide, >=98% (HPLC); NTZ;NSC 697855; HMS2051L04; HMS3393L04; HMS3655M11; HMS3715F10; Pharmakon1600-01503843; BCP13918; HY-B0217; ZINC3956788; Tox21_111018; Tox21_201226; BDBM50075050; CCG-35851; CN0040; MMV688991; NSC760057; STK395664; AKOS015915393; Tox21_111018_1; AC-1302; DB00507; KS-1160; MCULE-8701444531; NC00246; NSC-760057; NCGC00090774-02; NCGC00090774-03; NCGC00090774-04; NCGC00090774-05; NCGC00258778-01; AK-26130; BR-26130; NCI60_034935; AB0010620; FT-0601547; ST51059722; SW197626-2; 2-(5-nitrothiazol-2-ylcarbamoyl)phenyl acetate; D02486; J10428; S-3645; AB00639988-07; AB00639988-09; AB00639988_10; AB00639988_11; 981N094; A830877; Q-201475; Q2943789; NTZ; 2-(Acetyloxy)-N-(5-nitro-2-thiazolyl)benzamide; Salicylamide, N-(5-nitro-2-thiazolyl)- acetate (ester); Z1514087129; 2-[N-(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl acetate; [2-[(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl] ethanoate; Nitazoxanide, United States Pharmacopeia (USP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Irritable bowel syndrome | ICD-11: DD91 | [1] | ||
| PubChem CID | |||||
| Formula |
C12H9N3O5S
|
||||
| Canonical SMILES |
CC(=O)OC1=CC=CC=C1C(=O)NC2=NC=C(S2)[N+](=O)[O-]
|
||||
| InChI |
1S/C12H9N3O5S/c1-7(16)20-9-5-3-2-4-8(9)11(17)14-12-13-6-10(21-12)15(18)19/h2-6H,1H3,(H,13,14,17)
|
||||
| InChIKey |
YQNQNVDNTFHQSW-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=41684"></iframe>
|
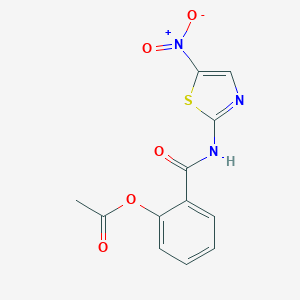 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 307.28 | Topological Polar Surface Area | 142 | |
| XlogP | 2 | Complexity | 428 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 4 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Nitazoxanide 500 mg tablet | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c yellow no. 10; Fd&c blue no. 2; Fd&c yellow no. 6; Magnesium stearate; Sucrose; Talc; Titanium dioxide; Lecithin, soybean; Polyvinyl alcohol; Hypromelloses; Sodium starch glycolate type a potato; Starch, corn; Xanthan gum
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Avera McKennan Hospital | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [3] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [3] | |||
| Polyvinyl alcohol | DIG Info | Debrisoquine 4-hydroxylase (EC50 = 354.8 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [6] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c yellow no. 10; Fd&c blue no. 2; Fd&c yellow no. 6; Magnesium stearate; Sucrose; Talc; Titanium dioxide; Lecithin, soybean; Polyvinyl alcohol; Hypromelloses; Sodium starch glycolate type a potato; Starch, corn; Xanthan gum
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Romark Laboratories | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [2] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [3] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [3] | |||
| Polyvinyl alcohol | DIG Info | Debrisoquine 4-hydroxylase (EC50 = 354.8 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Soybean lecithin | DIG Info | Albendazole monooxygenase (IC50 = 6.61 mg.mL-1) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
