| Synonyms |
Click to Show/Hide the Synonyms of This API
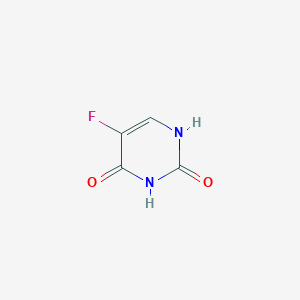
5-Fluorouracil; fluorouracil; 51-21-8; 5-FU; Fluoroplex; Efudex; Adrucil; Carac; 5-fluoropyrimidine-2,4(1H,3H)-dione; Fluracil; Fluoroblastin; Kecimeton; Carzonal; Timazin; Arumel; Efudix; Fluril; 5-Fluoracil; Fluracilum; Queroplex; Ulup; Phthoruracil; 5-fluoro-1H-pyrimidine-2,4-dione; Fluro Uracil; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; Fluorouracilum; Ftoruracil; Efurix; Fluri; 5 Fluorouracil; 5-Fluoropyrimidine-2,4-dione; Effluderm (free base); Fluorouracilo; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-; 2,4-Dihydroxy-5-fluoropyrimidine; Uracil, 5-fluoro-; 5-Fluoruracil; Ro 2-9757; 5-Fluor-2,4-pyrimidindiol; 5-fluoro uracil; 5-Ftouracyl; Fluorouracil, 5-; 5-fluoropyrimidine-2,4-diol; NSC 19893; NSC-19893; 5-Fluoro-2,4-pyrimidinedione; Fluorouracil (Adrucil); 5-Fluor-2,4-dihydroxypyrimidin; U-8953; 5-fluoro-uracil; Ro-2-9757; UNII-U3P01618RT; FU; 5-Fluorouracil, 99%; MFCD00006018; CHEMBL185; MLS000069498; 191047-65-1; 5FU; Fluroblastin; Phtoruracil; CHEBI:46345; Fluoro-uracile; Fluoro-uracilo; U3P01618RT; NSC19893; 5-fluoro-1,2,3,4-tetrahydropyrimidine-2,4-dione; 5-Faracil; Cinco FU; URF; NCGC00015442-02; Fluorouracile; Effluderm; SMR000038082; DSSTox_CID_634; Fluorouracile [DCIT]; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoruracil [German]; DSSTox_RID_75705; DSSTox_GSID_20634; Fluorouracilum [INN-Latin]; Fluorouracilo [INN-Spanish]; 5-Fluoropyrimidin-2,4-diol; 2,4-Dioxo-5-fluoropyrimidine; Fluorouracil Cream; CAS-51-21-8; Fluoroplex (TN); 5-Fluor-2,4-pyrimidindiol [Czech]; Adrucil (TN); 5-fluoro-1,3-dihydropyrimidine-2,4-dione; CCRIS 2582; Carac (TN); 5-Fluor-2,4-dihydroxypyrimidin [Czech]; HSDB 3228; SR-01000075881; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; EINECS 200-085-6; 5-Fluor-2,4(1H,3H)-pyrimidindion; Fluouracil; inhibits thymilidate synthetase; Tolak; 2,4-Dioxo-5-fluoropryimidine; 5-fluorourasil; AI3-25297; Fluoro Uracil; 5-florouracil; 5-fluorouacil; 5-flurouricil; 5-FU (TN); 5-Fluracil; 1upf; 5F-uracil; U 8953; 1-fluoro-1h-pyrimidine-2,4-dione; Adrucil (ICN); Fluorouracil (5-Fluoracil, 5-FU); Adrucil (Fluorouracil); Fluorouracil - Adrucil; Fluorouracil [USAN:USP:INN:BAN:JAN]; Spectrum_000841; ACMC-1ATRK; Opera_ID_134; Spectrum2_000076; Spectrum3_000434; Spectrum4_000557; Spectrum5_000718; WLN: T6MVMVJ EF; Lopac-F-6627; F0151; UPCMLD-DP130; EC 200-085-6; F 6627; SCHEMBL3646; 5-fluorpyrimidin-2,4-diol; Lopac0_000536; BSPBio_002048; KBioGR_001253; KBioSS_001321; MLS002415705; 5-fluoro-2,4-Pyrimidinediol; DivK1c_000054; SPECTRUM1500305; SPBio_000291; 5 FU; 5-fluoro-2,4-dioxo-pyrimidin; 5-fluoro-pyrimidine-2,4-diol; GTPL4789; DTXSID2020634; UPCMLD-DP130:001; Fluorouracil (JP17/USP/INN); HMS500C16; KBio1_000054; KBio2_001321; KBio2_003889; KBio2_006457; KBio3_001268; 5-Fluoro-2,3H)-pyrimidinedione; 2,4-Pyrimidinedione, 5-fluoro-; NINDS_000054; BCPP000428; HMS1920O18; HMS2090I04; HMS2091F19; HMS3259O03; HMS3261L13; HMS3654K22; HMS3715H03; HMS3865L03; Pharmakon1600-01500305; 5-Fluorouracil, analytical standard; BCP02083; 2,3H)-Pyrimidinedione, 5-fluoro-; Tox21_110150; Tox21_202335; Tox21_300112; Tox21_500536; ANW-31214; BBL009635; BDBM50340677; CCG-39879; CF0033; DL-399; NSC757036; Ro-29757; RW2456; s1209; SBB085751; STK297802; STL367375; ZINC38212689; AKOS000119162; AKOS003237897; AKOS008044307; Tox21_110150_1; BCP9000239; CS-0993; DB00544; KS-5129; LP00536; LS40596; MCULE-6338086431; NC00454; NSC-757036; SDCCGSBI-0050519.P005; IDI1_000054; NCGC00015442-01; NCGC00015442-03; NCGC00015442-04; NCGC00015442-05; NCGC00015442-06; NCGC00015442-07; NCGC00015442-08; NCGC00015442-09; NCGC00015442-10; NCGC00015442-11; NCGC00015442-12; NCGC00015442-15; NCGC00015442-16; NCGC00015442-30; NCGC00091349-01; NCGC00091349-02; NCGC00091349-03; NCGC00091349-04; NCGC00091349-05; NCGC00091349-07; NCGC00091349-08; NCGC00254023-01; NCGC00259884-01; NCGC00261221-01; 1004-03-1; 5-Fluoro-2,4-(1H,3H)-pyrimidinedione; AC-11201; AK-46307; HY-90006; NCI60_001652; SRI-10792-04; SRI-10792-05; SRI-10792-06; SRI-10792_07; SRI-10792_08; 5-Fluoro-1H-pyrimidine-2,4-dione(5FU); 5-Fluorouracil, >=99% (HPLC), powder; SBI-0050519.P004; 5-Fluoro-1H-pyrimidine-2,4-dione(5-FU); DB-051923; DB-065735; 5-Fluoro-1H-pyrimidine-2,4-dione (5-FU); AM20100252; EU-0100536; FT-0601511; FT-0668745; FT-0695666; FT-0695667; FT-0707652; ST45025877; SW199617-3; 5-Fluoro-1H-pyrimidine-2,4-dione(5-FUra); Fluorouracil, meets USP testing specifications; 51F218; 7375-EP2269989A1; 7375-EP2269994A1; 7375-EP2270008A1; 7375-EP2270018A1; 7375-EP2272827A1; 7375-EP2272832A1; 7375-EP2275102A1; 7375-EP2275412A1; 7375-EP2275413A1; 7375-EP2277876A1; 7375-EP2280012A2; 7375-EP2281563A1; 7375-EP2281815A1; 7375-EP2287156A1; 7375-EP2289892A1; 7375-EP2292233A2; 7375-EP2292614A1; 7375-EP2292615A1; 7375-EP2292617A1; 7375-EP2295416A2; 7375-EP2295426A1; 7375-EP2295427A1; 7375-EP2298748A2; 7375-EP2298768A1; 7375-EP2298772A1; 7375-EP2298780A1; 7375-EP2301928A1; 7375-EP2301933A1; 7375-EP2305219A1; 7375-EP2305243A1; 7375-EP2305640A2; 7375-EP2305642A2; 7375-EP2305671A1; 7375-EP2305689A1; 7375-EP2308833A2; 7375-EP2308839A1; 7375-EP2308855A1; 7375-EP2308861A1; 7375-EP2311807A1; 7375-EP2311808A1; 7375-EP2311825A1; 7375-EP2311827A1; 7375-EP2311829A1; 7375-EP2311840A1; 7375-EP2314590A1; 7375-EP2316459A1; 7375-EP2316831A1; 7375-EP2316834A1; 7375-EP2316974A1; 7375-EP2374454A1; C07649; D00584; W-5036; 29507-EP2270008A1; 29507-EP2270505A1; 29507-EP2272827A1; 29507-EP2289892A1; 29507-EP2292234A1; 29507-EP2292617A1; 29507-EP2295426A1; 29507-EP2295427A1; 29507-EP2298305A1; 29507-EP2308861A1; 29507-EP2311842A2; 42164-EP2272827A1; 42164-EP2275420A1; 42164-EP2277565A2; 42164-EP2277566A2; 42164-EP2277567A1; 42164-EP2277568A2; 42164-EP2277569A2; 42164-EP2277570A2; 42164-EP2277876A1; 42164-EP2292280A1; 42164-EP2292614A1; 42164-EP2295412A1; 42164-EP2295413A1; 42164-EP2295416A2; 42164-EP2298748A2; 42164-EP2298764A1; 42164-EP2298765A1; 42164-EP2298778A1; 42164-EP2305642A2; 42164-EP2308833A2; 42164-EP2311808A1; 42164-EP2311829A1; 42164-EP2311840A1; 5-Fluorouracil, Vetec(TM) reagent grade, >=99%; Q238512; W-60379; (5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione; 5-Fluoro-1H-pyrimidine-2,4-dione(5-fluoro uracil); SR-01000075881-1; SR-01000075881-3; SR-01000075881-5; W-202929; 5-Fluoro-1H-pyrimidine-2,4-dione (5-Fluorouracil); BRD-K24844714-001-02-1; Z275128052; 5-Fluoro-1H-pyrimidine-2,4-dione(5-fluorouracil)(5-FU); 5-Fluorouracil, certified reference material, TraceCERT(R); Fluorouracil, British Pharmacopoeia (BP) Reference Standard; Fluorouracil, European Pharmacopoeia (EP) Reference Standard; Fluorouracil, United States Pharmacopeia (USP) Reference Standard; pyrimidine antimetabolite: inhibits nucleic acid replication; tetratogen; Fluorouracil, Pharmaceutical Secondary Standard; Certified Reference Material
|
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM