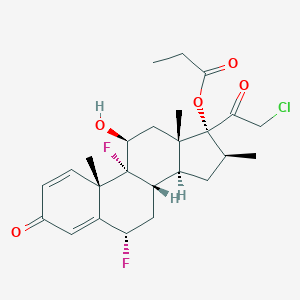
Details of the Active Pharmaceutical Ingredient (API)
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Halobetasol Propionate 0.05% lotion | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Diisopropyl Adipate; Octyldodecanol; Ceteth-20; Poloxamer 407; Cetyl Alcohol; Stearyl Alcohol; Glycerin; Carbomer Homopolymer Type C; Propylene; Glycol; Sodium; Hydroxide; Propylparaben; Butylparaben; Water
|
|||||
| Dosage Form | Lotion | |||||
| Company | Sun Pharmaceutical Industries | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Butylparaben | DIG Info | Estrogen receptor alpha (IC50 = 1420 nM) | [1] | |||
| Propylparaben sodium | DIG Info | Estrogen receptor alpha (EC50 = 38200 nM) | [2] | |||
| Diisopropyl adipate | DIG Info | Cholesterol 25-hydroxylase (IC50 = 110000 nM) | [3] | |||
| Cetyl alcohol | DIG Info | Breast cancer FM3A cells (EC50 = 1600 nM) | [4] | |||
| Polyoxyl 20 cetyl ether | DIG Info | Cholesterol 25-hydroxylase (EC50 = 211.5 uM) | [5] | |||
| Poloxamer 407 | DIG Info | Albendazole monooxygenase (EC50 = 32.8 uM) | [5] | |||
| Polyethylene glycol 4000 | DIG Info | Albendazole monooxygenase (Inhibition ratio < 40 %) | [6] | |||
| Halobetasol Propionate 0.01% lotion | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Carbomer Copolymer Type B; Carbomer Homopolymer Type A; Diethyl Sebacate; Edetate Disodium Dihydrate; Light Mineral Oil; Methylparaben; Propylparaben; Purified Water; Sodium Hydroxide; Sorbitan Monooleate; Sorbitol Solution
|
|||||
| Dosage Form | Lotion | |||||
| Company | Bausch Health Us | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| methylparaben | DIG Info | Carbonic anhydrase VII (Ki = 780 nM) | [7] | |||
| Propylparaben sodium | DIG Info | Estrogen receptor alpha (EC50 = 38200 nM) | [2] | |||
| Halobetasol Propionate 0.05% ointment | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Aluminum Stearate; Propylene Glycol; Petrolatum; White Wax; Sorbitan Sesquioleate; Monostearyl Citrate; Pentaerythritol
|
|||||
| Dosage Form | Ointment | |||||
| Company | Cosette Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Monostearyl citrate | DIG Info | Squalene synthase (IC50 = 9000 nM) | [8] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.