| Synonyms |
Click to Show/Hide the Synonyms of This API
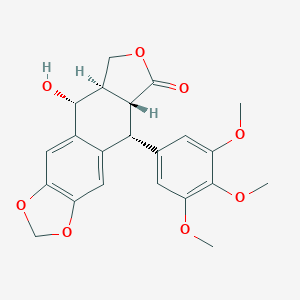
podophyllotoxin; Podofilox; 518-28-5; Condylox; Condyline; (-)-Podophyllotoxin; Wartec; Podophyllinic acid lactone; Podophyllotoxin 7; Warticon; Podophyllum; Podofilox [USAN]; UNII-L36H50F353; Podophyllotoxin, 95%; NSC24818; CHEMBL61; (5R,5aR,8aR,9R)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one; MLS000069495; CHEBI:50305; Podofilox (USAN); MFCD00075290; NSC-24818; L36H50F353; (10R,11R,15R,16R)-16-hydroxy-10-(3,4,5-trimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1(9),2,7-trien-12-one; NCGC00022001-05; Podofilm; SMR000059121; NSC 24818; DSSTox_CID_25645; DSSTox_RID_81023; DSSTox_GSID_45645; (5R,5aR,8aR,9R)-5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one; (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-isobenzofuro[5,6-f][1,3]benzodioxol-8-one; (5R,5aR,8aR,9R)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one; Podocon-25; Podophilox; (5R,5aR,8aR,9R)-5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one; (5R,9R,5aR,8aR)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,9,5a,8a-pentahydro-2H -isobenzofurano[5',6'-2,1]benzo[4,5-d]1,3-dioxolan-6-one; CCRIS 565; HSDB 7238; SR-05000001749; MLS002702981; EINECS 208-250-4; Podofillina; Podophylotoxin; AI3-50456; Mayapple isolate; Condylox (TN); CAS-518-28-5; Podophyllotoxin,(S); Prestwick_1018; Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-, (5R-(5alpha,5abeta,8aalpha,9alpha))-; Podofillina [Italian]; Podophyllotoxin (BAN); PubChem20017; Spectrum_000199; Opera_ID_1397; Prestwick0_000782; Prestwick1_000782; Prestwick2_000782; Prestwick3_000782; Spectrum2_000878; Spectrum4_000592; Spectrum5_001368; UPCMLD-DP035; SCHEMBL42243; BSPBio_000884; BSPBio_002352; KBioGR_001084; KBioSS_000679; MLS001148204; MLS002172467; MLS006010754; MLS006011412; BIDD:GT0123; DivK1c_000292; UNII-16902YVY2B; SPBio_000955; SPBio_002823; BPBio1_000974; CCRIS 4391; DTXSID3045645; UPCMLD-DP035:001; UPCMLD-DP035:002; BCBcMAP01_000165; HMS500O14; KBio1_000292; KBio2_000679; KBio2_003247; KBio2_005815; AMY2648; NINDS_000292; 16902YVY2B; HMS1570M06; HMS2093P16; HMS2097M06; HMS2235A23; HMS3259J19; HMS3714M06; Pharmakon1600-02300332; 9-Hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5ah)-one; ALBB-020906; BCP24085; Podophyllotoxin, analytical standard; ZINC3861806; EINECS 232-546-2; Tox21_110874; Tox21_202922; B18-5C; BBL033695; BDBM50035218; CCG-39894; CP0089; NSC759591; STK801918; AKOS000265559; Tox21_110874_1; AC-1656; CS-1185; DB01179; KS-1281; MCULE-9617461427; NC00675; ND-1185; NSC-759591; SDCCGMLS-0066888.P001; IDI1_000292; SMP1_000243; NCGC00022001-03; NCGC00022001-07; NCGC00022001-08; NCGC00022001-09; NCGC00022001-10; NCGC00022001-11; NCGC00022001-13; NCGC00022001-14; NCGC00260468-01; 1,3,3a,4,9,9a-Hexahydro-9-hydroxy-6,7-(methylenedioxy)-4-(3',4',5'-trimethoxyphenyl)benz(f)isobenzofuran-3-one; HY-15552; NCI60_001981; RD4-6269; ST066914; AB0012227; EN300-52746; C10874; D05529; P-6980; W-5067; 70339-EP2305243A1; 70339-EP2308833A2; 518P285; A828801; Q421193; SR-01000003030; SR-01000003030-3; SR-05000001749-1; SR-05000001749-2; BRD-K47869605-001-05-6; BRD-K47869605-001-18-9; Z1258578359; (10R,11R,15R,16R)-16-hydroxy-10-(3,4,5-trimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0?,?.0??,??]hexadeca-1(9),2,7-trien-12-one; (5R,5aR,8aR,9R)-5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-furo(3',4':6,7)naphtho[2,3-d]-1,3-dioxol-6(5aH)-one; (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-isobenzofuro[6,5-f][1,3]benzodioxol-8-one; (5R,5aR,8aR,9R)-5-oxidanyl-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one; 11016-28-7; 5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, [5R-(5.alpha.,5a.beta.,8a.alpha.,9.alpha.)]; furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-, (5R,5aR,8aR,9R)-; Furo[3',7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-, [5R-(5.alpha.,5a.beta.,8a.alpha.,9.alpha.)]-; Naphtho[2,3-dioxole-6-carboxylic acid, 5,6,7,8-tetrahydro-8-hydroxy-7-(hydroxymethyl)-5-(3,4,5-trimethoxyphenyl-, .gamma.-lactone
|
 click to show the detail info of this DFM
click to show the detail info of this DFM