Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00299) | |||||
|---|---|---|---|---|---|
| Name |
Gefitinib
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Gefitinib; 184475-35-2; Iressa; ZD1839; N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine; Irressat; gefitinib (zd1839); ZD 1839; ZD-1839; UNII-S65743JHBS; 4-(3'-Chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline; MFCD04307832; CHEMBL939; S65743JHBS; CHEBI:49668; NCGC00159455-02; DSSTox_CID_21034; DSSTox_RID_79614; DSSTox_GSID_41034; Iressa(TM); Iressa (TN); CCRIS 9011; CAS-184475-35-2; SR-00000000262; gefitinibum; Gefitinib (JAN/USAN/INN); Gefitinib [USAN:INN:BAN]; Gefitini; Iressa; gefitinib (iressa); Gefitinib - Iressa; IRRESSA; Iressa (AstraZeneca); nchembio866-comp14; Kinome_3321; Kinome_3322; Iressa; ZD 1839; SCHEMBL7866; Gefitinib,ZD-1839,Iressa; Gefitinib/ZD-1839,Iressa; KBioSS_002241; cc-596; MLS003899193; CU-00000000396-1; BDBM5447; cid_123631; EBD446; GTPL4941; DTXSID8041034; Gefitinib, >=98% (HPLC); AOB6917; QCR-105; SYN1042; BCPP000221; HMS2089B19; HMS3244M21; HMS3244M22; HMS3244N21; HMS3295A21; HMS3413H08; HMS3654A07; HMS3677H08; HMS3714A05; HMS3748E17; Pharmakon1600-01502274; BCP01365; Tox21_111683; ABP000855; CG0031; NSC715055; NSC759856; NSC800105; STK621310; ZINC19632614; AKOS000280752; Tox21_111683_1; AB20814; AC-1556; BCP9000718; CCG-220642; CS-0124; DB00317; KS-1204; MCULE-6951584187; NSC-715055; NSC-759856; NSC-800105; 4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxy-6-(3-morpholinopropoxy)quinazoline; 6-(3-morpholinopropoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine; N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine; NCGC00159455-03; NCGC00159455-04; NCGC00159455-05; NCGC00159455-06; NCGC00159455-08; NCGC00159455-09; NCGC00159455-14; AK-72948; BCB03_000781; BR-72948; HY-50895; SMR002204119; SY002154; AB0000095; AM20090619; FT-0602325; SW199108-4; EC-000.2409; D01977; G-4408; J10250; K00240; S-2176; 15215-EP2269994A1; 15215-EP2272827A1; 15215-EP2272832A1; 15215-EP2275413A1; 15215-EP2277865A1; 15215-EP2280006A1; 15215-EP2281813A1; 15215-EP2281815A1; 15215-EP2287156A1; 15215-EP2292234A1; 15215-EP2292615A1; 15215-EP2298768A1; 15215-EP2298778A1; 15215-EP2301533A1; 15215-EP2301933A1; 15215-EP2305640A2; 15215-EP2305671A1; 15215-EP2311807A1; 15215-EP2311827A1; 15215-EP2311840A1; 15215-EP2316832A1; 15215-EP2316833A1; 32333-EP2281815A1; 32333-EP2292615A1; 32333-EP2301933A1; 32333-EP2305640A2; 32333-EP2305671A1; 32333-EP2311827A1; AB01273954-01; AB01273954-02; AB01273954-03; AB01273954_04; 475G352; A812870; Q417824; Q-201149; SR-00000000262-2; SR-00000000262-3; Gefitinib, EuropePharmacopoeia (EP) Reference Standard; Z1551429741; (3-chloro-4-fluorophenyl)[7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-yl]amine; 4-(3'-chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)-quinazoline; Gefitinib for system suitability, EuropePharmacopoeia (EP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Lung cancer | ICD-11: 2C25 | [1] | ||
| PubChem CID | |||||
| Formula |
C22H24ClFN4O3
|
||||
| Canonical SMILES |
COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4
|
||||
| InChI |
1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27)
|
||||
| InChIKey |
XGALLCVXEZPNRQ-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=123631"></iframe>
|
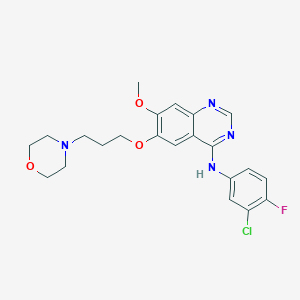 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 446.9 | Topological Polar Surface Area | 68.7 | |
| XlogP | 4.1 | Complexity | 545 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 8 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Gefitinib 250 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Ferric oxide red; Ferric oxide yellow; Titanium dioxide; Croscarmellose sodium; Polyethylene glycol 300; Cellulose, microcrystalline; Hypromelloses; Povidones
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | AstraZeneca | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [3] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [3] | |||
| Polyethylene glycol 300 | DIG Info | Multidrug resistance protein 1 (Inhibition ratio = 54 %) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
