Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00385) | |||||
|---|---|---|---|---|---|
| Name |
Mebendazole
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
mebendazole; 31431-39-7; Vermox; Telmin; Mebenvet; Pantelmin; Vermirax; Mebenoazole; Ovitelmin; Bantenol; Mebutar; Lomper; Besantin; Vermicidin; Verpanyl; Noverme; Mebendazol; Methyl (5-benzoyl-1H-benzo[d]imidazol-2-yl)carbamate; Mebendazolum; 5-Benzoyl-2-benzimidazolecarbamic acid methyl ester; Carbamic acid, (5-benzoyl-1H-benzimidazol-2-yl)-, methyl ester; Methyl 5-benzoyl-2-benzimidazolecarbamate; Methyl 5-benzoyl-2-benzimidazolylcarbamate; Methyl 5-benzoyl benzimidazole-2-carbamate; R 17635; methyl (5-benzoyl-1H-benzimidazol-2-yl)carbamate; (5-Benzoyl-1H-benzimidazol-2-yl)-carbamic acid methyl ester; R 17,635; Methyl N-(5-benzoyl-1H-benzimidazol-2-yl)carbamate; CCRIS 4479; methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate; MFCD00057872; methyl N-(6-benzoyl-1H-1,3-benzodiazol-2-yl)carbamate; NSC 184849; UNII-81G6I5V05I; 2-Benzimidazolecarbamic acid, 5-benzoyl-, methyl ester; CHEMBL685; N-2 (5-Benzoyl-benzimidazole) carbamate de methyle; N-(Benzoyl-5, benzimidazolyl)-2, carbamate de methyle; CHEBI:6704; Carbamic acid, N-(5-benzoylbenzimidazol-2-yl)-, methyl ester; Versid; Methyl 6-benzoyl-1H-benzimidazol-2-ylcarbamate; 81G6I5V05I; 5-Benzoyl-2-benzimidazolecarbamic acid, methyl ester; NSC184849; NSC-184849; methyl [5-(phenylcarbonyl)-1H-benzimidazol-2-yl]carbamate; NCGC00016806-01; (5-Benzoyl-1H-benzimidazol-2-yl)carbamic acid methyl ester; 2-Benzimidazolecarbamic acid, 5-benzoyl-, methyl ester (8CI); CAS-31431-39-7; Equivurm Plus; DSSTox_CID_20682; DSSTox_RID_79538; DSSTox_GSID_40682; Mebendazol [INN-Spanish]; Mebendazolum [INN-Latin]; Vermox (TN); Emverm; SMR000036734; HSDB 3232; Mebendazole Polymorph C; SR-01000003109; EINECS 250-635-4; Methyl N-(5-benzoyl-2-benzimidazolyl)carbamate; Mebatreat; Mebendazole (JAN/USP/INN); Equivurmp Plus; N-2 (5-Benzoyl-benzimidazole) carbamate de methyle [French]; Mebendazole,(S); N-(Benzoyl-5, benzimidazolyl)-2, carbamate de methyle [French]; Prestwick_310; Mebendazole [USAN:USP:INN:BAN:JAN]; Spectrum_001298; CPD000036734; Prestwick0_000217; Prestwick1_000217; Prestwick2_000217; Prestwick3_000217; Spectrum2_001401; Spectrum3_001439; Spectrum4_000416; Spectrum5_001381; Probes1_000013; Probes2_000149; Cambridge id 5250893; Methyl [5-(Benzoyl)benzimidazol-2-yl]carbamate; TimTec1_000869; Oprea1_278237; Oprea1_768530; R-17635; SCHEMBL15860; BSPBio_000233; BSPBio_003178; CBDivE_010559; KBioGR_000712; KBioSS_001778; MLS000028491; MLS006011879; BIDD:GT0087; DivK1c_000751; SPECTRUM1501110; SPBio_001442; SPBio_002154; BPBio1_000257; DTXSID4040682; HMS502F13; KBio1_000751; KBio2_001778; KBio2_004346; KBio2_006914; KBio3_002398; NINDS_000751; HMS1536H11; HMS1568L15; HMS1921F03; HMS2090B03; HMS2092B15; HMS2095L15; HMS3259B11; HMS3604N11; HMS3712L15; N-(6-benzoyl-1H-benzimidazol-2-yl)carbamic acid methyl ester; Pharmakon1600-01501110; ZINC121541; EBD12117; Tox21_110620; ANW-54402; BBL008298; BDBM50180753; CCG-39628; MMV003152; NSC757838; SBB057003; STK093862; AKOS000539066; AKOS015896232; Carbamic acid, (5-benzoyl-1H-benzimidazol-2-yl)-, methyl ester (9CI); Tox21_110620_1; CS-3974; DB00643; MCULE-4133611535; NC00639; NE41992; NSC-757838; IDI1_000751; NCGC00016806-02; NCGC00016806-03; NCGC00016806-04; NCGC00016806-05; NCGC00016806-06; NCGC00016806-07; NCGC00016806-08; NCGC00016806-09; NCGC00016806-10; NCGC00016806-12; NCGC00016806-13; NCGC00021698-03; NCGC00021698-04; NCGC00021698-05; NCGC00021698-06; NCGC00021698-07; AC-12064; AK-97348; AS-12272; HY-17595; ST011967; SY051142; SBI-0051641.P002; AB00052203; FT-0628179; FT-0628180; EN300-50844; D00368; W-6804; 5-Benzoyl-2-benzimidazolylcarbamicacidmethylester; AB00052203-09; AB00052203_10; Mebendazole, VETRANAL(TM), analytical standard; 431M397; A820852; AG-205/04588045; Mebendazole, analytical standard, >=98% (HPLC); Methyl (6-benzoyl-1H-benzimidazol-2-yl)carbamate; Q422194; methyl 5-benzoyl-1H-benzo[d]imidazol-2-ylcarbamate; SR-01000003109-2; SR-01000003109-3; W-106901; BRD-K77987382-001-01-7; BRD-K77987382-001-06-6; BRD-K77987382-001-08-2; methyl N-(5-benzoyl-1H-1,3-benzimidazol-2-yl)carbamate; Z234895185; 5-benzoyl-1H-benzimidazol-2-yl carbamic acid methyl ester; Mebendazole, European Pharmacopoeia (EP) Reference Standard; methoxy-N-[5-(phenylcarbonyl)benzimidazol-2-yl]carboxamide; methoxy-N-[6-(phenylcarbonyl)benzimidazol-2-yl]carboxamide; methyl N-[6-(phenylcarbonyl)-1H-benzimidazol-2-yl]carbamate; (5-benzoyl-1(3)H-benzoimidazol-2-yl)-carbamic acid methyl ester;; Mebendazole, United States Pharmacopeia (USP) Reference Standard; Mebendazole Polymorph C, United States Pharmacopeia (USP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Ascariasis | ICD-11: 1F62 | [1] | ||
| PubChem CID | |||||
| Formula |
C16H13N3O3
|
||||
| Canonical SMILES |
COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CC=C3
|
||||
| InChI |
1S/C16H13N3O3/c1-22-16(21)19-15-17-12-8-7-11(9-13(12)18-15)14(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,17,18,19,21)
|
||||
| InChIKey |
OPXLLQIJSORQAM-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4030"></iframe>
|
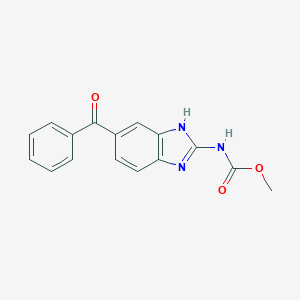 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 295.29 | Topological Polar Surface Area | 84.1 | |
| XlogP | 2.8 | Complexity | 423 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 4 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Mebendazole 100 mg tablet | Click to Show/Hide the Full List of Formulation(s): 4 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Anhydrous lactose; Sodium lauryl sulfate; Saccharin sodium dihydrate; Fd&c yellow no. 6; Magnesium stearate; Cellulose, microcrystalline; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | Bryant Ranch Prepack | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Saccharin sodium dihydrate | DIG Info | Carbonic anhydrase XII (Ki = 633 nM) | [2] | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Anhydrous lactose; Sodium lauryl sulfate; Fd&c yellow no. 6; Magnesium stearate; Cellulose, microcrystalline; Saccharin sodium; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | Physicians Total Care; Teva Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Drug Formulation 3 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Stearic acid; Anhydrous lactose; Sodium lauryl sulfate; Fd&c yellow no. 6; Magnesium stearate; Cellulose, microcrystalline; Saccharin sodium; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | Amedra Pharmaceuticals; Impax Specialty Pharma | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Stearic acid | DIG Info | Phosphodiesterase 3A (IC50 = 3.1 uM) | [3] | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Drug Formulation 4 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sodium lauryl sulfate; Fd&c yellow no. 6; Magnesium stearate; Talc; Silicon dioxide; Microcrystalline cellulose; Orange; Saccharin sodium; Sodium starch glycolate type a potato; Starch, corn
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | Johnson & Johnson | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sunset yellow FCF | DIG Info | Solute carrier SLCO2B1 (Ki = 68.4 uM) | [4] | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Mebendazole 500 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Sucralose; Magnesium stearate; Water; Crospovidone (15 mpa.s at 5%); Microcrystalline cellulose; Povidone, unspecified; Strawberry
|
|||||
| Dosage Form | Chewable Tablet | |||||
| Company | Janssen Pharmaceutical | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sucralose | DIG Info | Carbonic anhydrase II (Ki = 300 nM) | [6] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
