Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00610) | |||||
|---|---|---|---|---|---|
| Name |
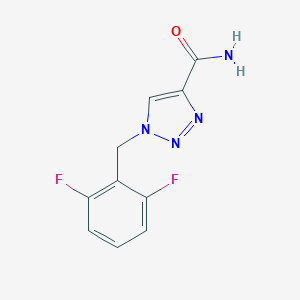
Rufinamide
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Rufinamide; 106308-44-5; Inovelon; Banzel; 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide; Cgp 33101; CGP-33101; 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-Triazole-4-carboxamide; RUF 331; RUF-331; UNII-WFW942PR79; C10H8F2N4O; 1-[(2,6-difluorophenyl)methyl]triazole-4-carboxamide; E 2080; E-2080; 1H-1,2,3-Triazole-4-carboxamide, 1-[(2,6-difluorophenyl)methyl]-; WFW942PR79; MFCD00865314; NCGC00165883-02; DSSTox_CID_26506; DSSTox_RID_81675; DSSTox_GSID_46506; 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-triazole-4 carboxamide; 1H-1,2,3-Triazole-4-carboxamide, 1-((2,6-difluorophenyl)methyl)-; SMR000857122; CAS-106308-44-5; Rufinamide [USAN:INN:BAN]; SYN111; SYN-111; CGP33101; Banzel (TN); Rufinamide (Banzel); CGP 33,101; PubChem14896; Rufinamide (JAN/USP/INN); MLS001332513; MLS001332514; SCHEMBL230448; GTPL7470; ZINC7782; CHEMBL1201754; DTXSID1046506; CHEBI:134966; HMS2232M19; HMS3262O14; HMS3371A06; HMS3651O05; HMS3884G07; BCP21828; HY-A0042; Tox21 112267; Tox21_112267; Tox21_500796; BDBM50515492; AKOS005145897; Rufinamide, >=98% (HPLC), powder; Tox21_112267_1; AC-1429; AS07135; CCG-222100; CS-1455; DB06201; LP00796; MCULE-6872329028; SB18904; SDCCGSBI-0633757.P001; NCGC00165883-01; NCGC00165883-03; NCGC00165883-04; NCGC00165883-11; NCGC00261481-01; AS-13861; AB0017630; AK00592772; FT-0656828; FT-0674479; SW219770-1; 1-(2,6-difluorobenzyl)triazole-4-carboxamide; D05775; J10459; W-5135; AB00918347-05; AB00918347_06; 308R445; A801414; Q408565; SR-01000842156; J-001568; SR-01000842156-4; 1-[(2,6-difluorophenyl)methyl]-4-triazolecarboxamide; F0001-2404; Z1541638521; 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazol-4-carboxamide; Rufinamide, United States Pharmacopeia (USP) Reference Standard; 1-[[2,6-bis(fluoranyl)phenyl]methyl]-1,2,3-triazole-4-carboxamide
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Lennox-gastaut syndrome | ICD-11: 8A62 | [1] | ||
| PubChem CID | |||||
| Formula |
C10H8F2N4O
|
||||
| Canonical SMILES |
C1=CC(=C(C(=C1)F)CN2C=C(N=N2)C(=O)N)F
|
||||
| InChI |
1S/C10H8F2N4O/c11-7-2-1-3-8(12)6(7)4-16-5-9(10(13)17)14-15-16/h1-3,5H,4H2,(H2,13,17)
|
||||
| InChIKey |
POGQSBRIGCQNEG-UHFFFAOYSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=129228"></iframe>
|
 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 238.19 | Topological Polar Surface Area | 73.8 | |
| XlogP | 0.7 | Complexity | 282 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 3 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Rufinamide 200 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Ferric oxide red; Talc; Titanium dioxide; Carboxymethylcellulose sodium; Silicon dioxide; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycols; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Eisai | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Carboxymethylcellulose sodium | DIG Info | Albendazole monooxygenase (EC50 = 12.6 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Rufinamide 400 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Ferric oxide red; Talc; Titanium dioxide; Carboxymethylcellulose sodium; Silicon dioxide; Cellulose, microcrystalline; Hypromelloses; Polyethylene glycols; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Eisai | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| Carboxymethylcellulose sodium | DIG Info | Albendazole monooxygenase (EC50 = 12.6 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
| Silicon dioxide | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
