| Synonyms |
Click to Show/Hide the Synonyms of This API
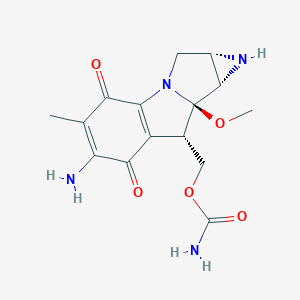
mitomycin C; Mitomycin; 50-07-7; Mutamycin; Ametycine; Mitomycin-C; Mitocin-C; Ametycin; Mytomycin; Mitomycinum; Mytozytrex; Mitozytrex; Mitocin C; Mitamycin; MMC; Mitosol; 7-Amino-9alpha-methoxymitosane; NSC-26980; C15H18N4O5; NSC 26980; Mitomycyna C; NCI-C04706; Mito-C; NSC26980; Mit-C; Mitomycine; UNII-50SG953SK6; Mitomycinum C; Mitomycins; CHEBI:27504; RCRA waste number U010; 50SG953SK6; Mitomycyna C [Polish]; MFCD00078109; ((1aS,8S,8aR,8bS)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2',3':3,4]pyrrolo[1,2-a]indol-8-yl)methyl carbamate; Mitomicina; DSSTox_CID_898; DSSTox_RID_75853; DSSTox_GSID_20898; Mitomycin (TN); Mitomycine [INN-French]; Mitomycinum [INN-Latin]; [(1aS,8S,8aR,8bS)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamate; Azirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1aS,8S,8aR,8bS)-; Mitomicina [INN-Spanish]; CAS-50-07-7; Muamycin (TN); [1aS-(1a?,8?,8a?,8b?)]-6-Amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methylazirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione; Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1aS,8S,8aR,8bS)-; CCRIS 414; HSDB 3239; Mitomycin (USP/INN); 6-Amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a-methoxy-5-methylazirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione carbamate (ester); MLS002702984; (amino-methoxy-methyl-dioxo-[?]yl)methyl carbamate; EINECS 200-008-6; Mitomycin C, Streptomyces caespitosus; RCRA waste no. U010; 7-Amino-9.alpha.-methoxymitosane; Mitonco; Mitoplus; MitoExtra; AI3-26199; Mitomycin [USAN:USP:INN:BAN]; NCGC00095258-01; [(1aS,8S,8aR,8bS)-6-Amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2'',3'':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamate; [(1aS,8S,8aR,8bS)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamate; Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a-methoxy-5-methyl-, carbamate (ester); Jelmyto (TN); Mitomycin C, Streptomyces caespitosus, Carrier-Free; Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1aS-(1aalpha,8beta,8aalpha,8balpha))-; Mitomycin C from Streptomyces caespitosus; Mitomycin C (JP17); CHEMBL105; SCHEMBL3760; CBiol_001927; BSPBio_001267; KBioGR_000607; KBioSS_000607; 1404-00-8; MLS001332654; Mitozytrex (TN) (Supergene); AMETYCIN pound notmitomycin C; GTPL7089; DTXSID2020898; KBio2_000607; KBio2_003175; KBio2_005743; KBio3_001073; KBio3_001074; EX-A501; UGN-101; UGN-102; BCPP000410; Bio1_000213; Bio1_000702; Bio1_001191; Bio2_000464; Bio2_000944; HMS1362O09; HMS1792O09; HMS1990O09; HMS2089F16; HMS3403O09; AMY10316; Tox21_111493; AC-918; BDBM50428658; GR-311; s8146; ZINC30726187; AKOS015895703; Tox21_111493_1; ACN-038344; BCP9000285; CCG-208564; CS-0564; DB00305; KS-5148; IDI1_002219; SMP1_000307; NCGC00163468-02; NCGC00163468-03; NCGC00163468-05; NCGC00163468-06; Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1aS-(1aalpha,8beta,8aalpha,8balpha))- (9CI); HY-13316; SMR000058401; BCP0726000181; M2320; C06681; D00208; W-5071; AB00918689-03; AB00918689-04; 078M109; Mitomycin C, Antibiotic for Culture Media Use Only; Q-201410; BRD-K59670716-001-02-6; BRD-K59670716-001-06-7; Q19856779; Mitomycin C, contains 2 mg Mitomycin C and 48 mg NaCl; UNII-V03E10691T component NWIBSHFKIJFRCO-WUDYKRTCSA-N; WLN: T D3 B556 BN EM JV MVTTT&J GO1 H1OVZ KZ L1; Mitomycin C from Streptomyces caespitosus, >=970 mug/mg (USP XXIV); Mitomycin C from Streptomyces caespitosus, powder, BioReagent, suitable for cell culture; Mitomycin C from Streptomyces caespitosus, powder, contains NaCl as solubilizer; [(4S,6S,7R,8S)-11-amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.0^{2,7}.0^{4,6}]trideca-1(9),11-dien-8-yl]methyl carbamate; [1aS-(1aalpha,8beta,8aalpha,8balpha)]-6-Amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methylazirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione; Azirino[2',4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a- methoxy-5-methyl-, carbamate (ester); Azirino[2',4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b- hexahydro-8a-methoxy-5-methyl-, [1aR-(1a.alpha.,8.beta.,8a.alpha.,8b.alpha.)]-; Mitomycin C from Streptomyces caespitosus, >=98% (HPLC), potency: >=970 mug per mg (USP XXIV), gamma-irradiated, suitable for cell culture
|
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM