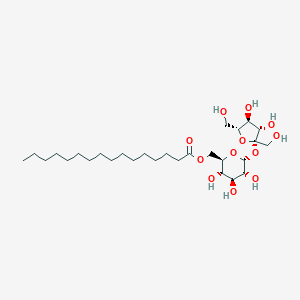
Details of the Drug Inactive Ingredient (DIG)
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| Oxidoreductase (ORase) | ||||||
| DBT Name: Albendazole monooxygenase (CYP3A4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 8.8 uM (tested by experiment) | [1] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CP3A4_HUMAN | |||||
| DBT Name: Cytochrome P450 2C9 (CYP2C9) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 559 uM (tested by experiment) | [1] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CP2C9_HUMAN | |||||
| DBT Name: Mephenytoin 4-hydroxylase (CYP2C19) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 119.1 uM (tested by experiment) | [1] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CP2CJ_HUMAN | |||||
| DBT Name: Cytochrome P450 3A5 (CYP3A5) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | EC50 = 6.4 uM (tested by experiment) | [1] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CP3A5_HUMAN | |||||
| Cell line (CELL) | ||||||
| DBT Name: Colon cancer HCT-15 cells (HCT-15) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | IC50 = 0.65 ug.mL-1 (estimated based on the structural similarity with CHEMBL552345 ) | [2] | ||||
| Structural Similarity | Tanimoto coefficient = 0.940298507 | |||||
| Tested Species | Homo sapiens (Human) | |||||
| Cellosaurus ID | CVCL_0292 | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.