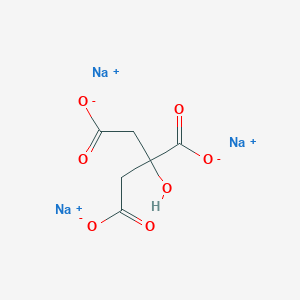
Details of the Drug Inactive Ingredient (DIG)
| Full List of Active Pharmaceutical Ingredients (APIs) Co-administrated with This DIG | ||||||
|---|---|---|---|---|---|---|
| ICD Disease Classification 01 | Infectious/parasitic disease | Click to Show/Hide | ||||
|
Penicillin V
|
API Info |
Pulmonary tuberculosis [ICD-11: 1B10]
|
[1] | |||
| ICD Disease Classification 02 | Benign/in-situ/malignant neoplasm | Click to Show/Hide | ||||
|
Etoposide
|
API Info |
Testicular carcinoma [ICD-11: 2C80]
|
[2] | |||
| ICD Disease Classification 05 | Endocrine/nutritional/metabolic disease | Click to Show/Hide | ||||
|
Rosuvastatin
|
API Info |
Hypertriglyceridaemia [ICD-11: 5C80]
|
[3] | |||
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | ||||
|
Quetiapine
|
API Info |
Schizophrenia [ICD-11: 6A20]
|
[4] | |||
|
Buprenorphine
|
API Info |
Opiate dependence [ICD-11: 6C43]
|
[5] | |||
| ICD Disease Classification 08 | Nervous system disease | Click to Show/Hide | ||||
|
Acetaminophen
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[6] | |||
|
Pramipexole
|
API Info |
Parkinsonism [ICD-11: 8A00]
|
[7] | |||
| ICD Disease Classification 09 | Visual system disease | Click to Show/Hide | ||||
|
Dextromethorphan
|
API Info |
Allergic conjunctivitis [ICD-11: 9A60]
|
[8] | |||
| ICD Disease Classification 10 | Ear/mastoid process disease | Click to Show/Hide | ||||
|
Amoxicillin
|
API Info |
Acute otitis media [ICD-11: AB00]
|
[9] | |||
| ICD Disease Classification 11 | Circulatory system disease | Click to Show/Hide | ||||
|
Carvedilol
|
API Info |
Congestive heart failure [ICD-11: BD10]
|
[10] | |||
| ICD Disease Classification 15 | Musculoskeletal/connective-tissue disease | Click to Show/Hide | ||||
|
Meloxicam
|
API Info |
Rheumatoid arthritis [ICD-11: FA20]
|
[11] | |||
|
Flurbiprofen
|
API Info |
Rheumatoid arthritis [ICD-11: FA20]
|
[12] | |||
| ICD Disease Classification 16 | Genitourinary system disease | Click to Show/Hide | ||||
|
Ibuprofen
|
API Info |
Dysmenorrhea [ICD-11: GA34]
|
[13] | |||
|
Oxybutynin
|
API Info |
Overactive bladder [ICD-11: GC50]
|
[14] | |||
|
Erythromycin
|
API Info |
Pelvic inflammatory disease [ICD-11: GA05]
|
[15] | |||
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| Lyase/isomerase/ligase (L/I/G) | ||||||
| DBT Name: Carbonic anhydrase IV (CA4) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 99 nM (tested by experiment) | [16] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CAH4_HUMAN | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.