| Synonyms |
Click to Show/Hide the Synonyms of This DIG
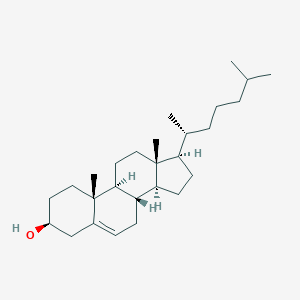
cholesterol; 57-88-5; Cholesterin; Cholesteryl alcohol; Cholest-5-en-3beta-ol; Cholestrin; Cordulan; Dusoline; Dusoran; Cholesterine; Cholestrol; Hydrocerin; Dythol; Kathro; Lanol; Super hartolan; Provitamin D; Cholesterol base H; Lidinite; Lidinit; Nimco cholesterol base H; 5-Cholesten-3beta-ol; Wool alcohols B. P.; (-)-Cholesterol; (3beta)-cholest-5-en-3-ol; Tegolan (VAN); 5:6-Cholesten-3beta-ol; Cholest-5-en-3-beta-ol; Tegolan; 3beta-Hydroxycholest-5-ene; Cholest-5-en-3-ol (3beta)-; Cholest-5-en-3-ol; 3-beta-Hydroxycholest-5-ene; CCRIS 2834; Dastar; HSDB 7106; delta(sup 5)-Cholesten-3-beta-ol; Fancol CH; 3beta-Hydroxy-5-cholestene; NSC 8798; UNII-97C5T2UQ7J; 5-Cholesten-3B-ol; CHEBI:16113; AI3-03112; Nimco cholesterol base No. 712; 5:6-Cholesten-3-ol; MFCD00003646; 5-Cholesten-3.beta.-ol; Cholest-5-en-3.beta.-ol; 5,6-Cholesten-3.beta.-ol; 3.beta.-Hydroxycholest-5-ene; 97C5T2UQ7J; (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3beta,14beta,17alpha)-cholest-5-en-3-ol; NSC8798; NSC-8798; NCGC00159351-02; Cholest-5-en-3-ol (3.beta.)-; (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol; (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol; Cholesterinum; Cholesterol-t; 3h-cholesterol; (3S,8S,9S,10R,13R,14S,17R)-17-[(1R)-1,5-dimethylhexyl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3H)-Cholesterol; Cholest-5-en-3-ol, (3beta)-; EINECS 200-353-2; epicholesterin; Cholesterol [BAN:JAN:NF]; .DELTA.5-Cholesten-3-.beta.-ol; cholesterol group; 1zhy; Cholesterol,(S); Cholest-5-en-3-ol, (3.beta.)- #; Cholesterol (TN); sterol;Cholesterin,Cholest-5-en-3beta-ol; 20-epi-cholesterol; 20-iso-cholesterol; Cholesterol, 94%; Liquid crystal CN/9; (+)-ent-Cholesterol; cholest-5-en-3b-ol; 5-Cholesten-3ss--ol; 5-cholestene-3beta-ol; PubChem19974; 5-Cholesten-3bet.-ol; Cholesterol, 99.0%; Cholesterol (JP17/NF); DSSTox_CID_2401; Phospholipon & Cholesterol; 3ss-Cholest-5-en-3-ol; Cholesterol, Plant-Derived; Delta5-Cholesten-3beta-ol; Epitope ID:150761; EC 200-353-2; 3beta-cholest-5-en-3-ol; 3ss--Hydroxy-5-cholestene; 3bet.-Hydroxy-5-cholestene; DSSTox_RID_76573; BIDD:PXR0095; DSSTox_GSID_22401; (3b)-cholest-5-en-3-ol; 5:6-Cholesten-3.beta.-ol; 20bFH-cholest-5-en-3b-ol; BIDD:ER0548; Cholest-5-en-3-ol (3beta)-, labeled with tritium; CHEMBL112570; GTPL2718; DTXSID3022401; (3 )-Cholest-5-en-3-ol; BDBM20192; (20bFH)-cholest-5-en-3b-ol; CHEBI:140435; 10,13-dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; Cholesterol, Sigma Grade, >=99%; HY-N0322; ZINC3875383; Tox21_111595; ANW-32821; CMC_13392; LMST01010001; s4154; SBB058670; AKOS005267135; Cholest-5-en-3-ol, (3-.beta.)-; CS-5106; DB04540; GS-6857; MCULE-6413140986; CAS-57-88-5; Cholesterol, NIST(R) SRM(R) 911c; SMP1_000069; NCGC00159351-03; 22243-67-0; 80356-14-5; Cholesterol 10 microg/mL in Acetonitrile; Cholesterol, tested according to Ph.Eur.; Cholesterol, from lanolin, >=95.0% (GC); Cholesterol, from lanolin, >=99.0% (GC); 1889-EP2269610A2; 1889-EP2269977A2; 1889-EP2269989A1; 1889-EP2269990A1; 1889-EP2270011A1; 1889-EP2270505A1; 1889-EP2272822A1; 1889-EP2272825A2; 1889-EP2272834A1; 1889-EP2272841A1; 1889-EP2272843A1; 1889-EP2274983A1; 1889-EP2275105A1; 1889-EP2277507A1; 1889-EP2277565A2; 1889-EP2277566A2; 1889-EP2277567A1; 1889-EP2277568A2; 1889-EP2277569A2; 1889-EP2277570A2; 1889-EP2277865A1; 1889-EP2280001A1; 1889-EP2280282A1; 1889-EP2281817A1; 1889-EP2281824A1; 1889-EP2281899A2; 1889-EP2284157A1; 1889-EP2284158A1; 1889-EP2286795A1; 1889-EP2287165A2; 1889-EP2287166A2; 1889-EP2289510A1; 1889-EP2289883A1; 1889-EP2292228A1; 1889-EP2292234A1; 1889-EP2292280A1; 1889-EP2292596A2; 1889-EP2292612A2; 1889-EP2292620A2; 1889-EP2295406A1; 1889-EP2295409A1; 1889-EP2295411A1; 1889-EP2295417A1; 1889-EP2295429A1; 1889-EP2295439A1; 1889-EP2298312A1; 1889-EP2298728A1; 1889-EP2298731A1; 1889-EP2298735A1; 1889-EP2298742A1; 1889-EP2298745A1; 1889-EP2298747A1; 1889-EP2298772A1; 1889-EP2298776A1; 1889-EP2298779A1; 1889-EP2301923A1; 1889-EP2301929A1; 1889-EP2301935A1; 1889-EP2301936A1; 1889-EP2305648A1; 1889-EP2305668A1; 1889-EP2305674A1; 1889-EP2305695A2; 1889-EP2305696A2; 1889-EP2305697A2; 1889-EP2305698A2; 1889-EP2305825A1; 1889-EP2308479A2; 1889-EP2308838A1; 1889-EP2308839A1; 1889-EP2308858A1; 1889-EP2309584A1; 1889-EP2311811A1; 1889-EP2311816A1; 1889-EP2311817A1; 1889-EP2311821A1; 1889-EP2311823A1; 1889-EP2311825A1; 1889-EP2311836A1; 1889-EP2311841A1; 1889-EP2314295A1; 1889-EP2314576A1; 1889-EP2314588A1; 1889-EP2314593A1; 1889-EP2316457A1; 1889-EP2316458A1; 1889-EP2316470A2; 1889-EP2316825A1; 1889-EP2316826A1; 1889-EP2316827A1; 1889-EP2316828A1; 1889-EP2377510A1; C00187; D00040; Q43656; Z-0798; AB00443913_03; Cholesterol, >=95% (GC), powder, Ash, free; Cholesterol, Vetec(TM) reagent grade, >=92.5%; Soya phospholipon & Cholesterol (2:1 molar ratio); W-105431; 5BBA213B-ECF4-44AF-8AAF-8A0120F2F886; Cholesterol (cGMP, animal-origin free, vegetal-derived); Cholesterol, from sheep wool, >=92.5% (GC), powder; WLN: L E5 B666 LUTJ A1 E1 FY1&3Y1&1 OQ; Cholesterol (non-cGMP, animal-origin free, vegetal-derived); Cholesterol, European Pharmacopoeia (EP) Reference Standard; Cholesterol, powder, BioReagent, suitable for cell culture, >=99%; Cholesterol, United States Pharmacopeia (USP) Reference Standard; Cholesterol solution, certified reference material, 10 mg/mL in chloroform; Cholesterol, Pharmaceutical Secondary Standard; Certified Reference Material; PhytoChol Puriss (animal-origin free, vegetal-derived Cholesterol); Cholesterol, from sheep wool, Controlled origin, meets USP/NF testing specifications; (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadec-7-en-5-ol; 14-((1R)-1,5-dimethylhexyl)(1S,5S,10S,11S,2R,14R,15R)-2,15-dimethyltetracyclo[ 8.7.0.0<2,7>.0<11,15>]heptadec-7-en-5-ol; Cholesterol, Plant-Derived, SyntheChol(R), PharmaGrade, USP/NF, Ph Eur, Manufactured under appropriate GMP controls for pharma or biopharmaceutical production; SyntheChol(R) NS0 Supplement, 500 x, synthetic cholesterol, animal component-free, aqueous solution, sterile-filtered, suitable for cell culture
|
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT
 click to show the detail info of this DBT
click to show the detail info of this DBT