Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00227) | |||||
|---|---|---|---|---|---|
| Name |
Elvitegravir
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Elvitegravir; 697761-98-1; GS-9137; JTK-303; GS 9137; Elvitegravir (GS-9137); UNII-4GDQ854U53; (S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-methylbutan-2-yl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; CHEBI:72289; CHEMBL204656; 6-(3-Chloro-2-fluorobenzyl)-1-[1(S)-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid; 4GDQ854U53; 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; Vitekta; 6-(3-Chloro-2-fluorobenzyl)-1-((2S)-1-hydroxy-3-methylbutan-2-yl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 6-(3-chloro-2-fluorobenzyl)-1-[(1S)-1-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; JTK 303; Elvitegravir [USAN:INN]; elvitegravirum; 6-[(3-chloro-2-fluoro-phenyl)methyl]-1-[(1S)-1-(hydroxymethyl)-2-methyl-propyl]-7-methoxy-4-oxo-quinoline-3-carboxylic acid; Vitekta (TN); Elvitegravir (GS-9137, JTK-303); PubChem19145; Elvitegravir (JAN/USAN); C23H23CLFNO5; cc-521; MLS006011136; SCHEMBL726252; AOB6957; BCPP000242; EX-A1542; ABP000243; BDBM50183273; MFCD11846134; ZINC13682481; Elvitegravir; GS9137; JTK 303; AKOS025396642; BCP9000642; CCG-269208; CS-0439; DB09101; SB16498; NCGC00346565-01; NCGC00346565-04; AC-29947; AS-16986; Elvitegravir,EVG,GS-9137,JTK-303/; HY-14740; QC-10447; SMR004702914; AB0008021; Elvitegravir 100 microg/mL in Acetonitrile; SW219721-1; EC-000.2332; D06677; AB01274749-01; AB01274749_02; J-518006; Q2740966; BRD-K54472332-001-01-8; 3-Quinolinecarboxylic acid, 6-((3-chloro-2-fluorophenyl)methyl)-1,4-dihydro-1-((1S)-1-(hydroxymethyl)-2-methylpropyl)-7-methoxy-4-oxo-; 3-Quinolinecarboxylic acid, 6-[(3-chloro-2-fluorophenyl)methyl]-1,4-dihydro-1-[(1S)-1-isopropyl-2-hydroxyethyl]-7-methoxy-4-oxo-; 6-(3-chloro-2-fluorobenzyl)-1-[(S)-1-hydroxymethyl-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Human immunodeficiency virus infection | ICD-11: 1C60 | [1] | ||
| PubChem CID | |||||
| Formula |
C23H23ClFNO5
|
||||
| Canonical SMILES |
CC(C)[C@@H](CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O
|
||||
| InChI |
1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1
|
||||
| InChIKey |
JUZYLCPPVHEVSV-LJQANCHMSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5277135"></iframe>
|
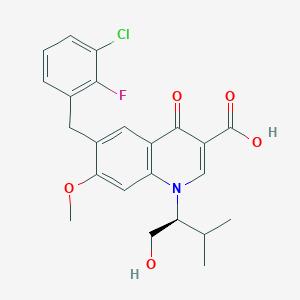 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 447.9 | Topological Polar Surface Area | 87.1 | |
| XlogP | 5.3 | Complexity | 702 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 7 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Elvitegravir 150 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Fd&c blue no. 2; Magnesium stearate; Ferric oxide yellow; Talc; Titanium dioxide; Water; Croscarmellose sodium; Polyethylene glycol 3350; Polyvinyl alcohol; Cellulose, microcrystalline; Hydroxypropyl cellulose (type h)
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Gilead Sciences | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [3] | |||
| Polyvinyl alcohol | DIG Info | Debrisoquine 4-hydroxylase (EC50 = 354.8 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [5] | |||
| Polyethylene glycol 3350 | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [5] | |||
| Elvitegravir 85 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Fd&c blue no. 2; Magnesium stearate; Ferric oxide yellow; Talc; Titanium dioxide; Water; Croscarmellose sodium; Polyethylene glycol 3350; Polyvinyl alcohol; Cellulose, microcrystalline; Hydroxypropyl cellulose (type h)
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Gilead Sciences | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [2] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [3] | |||
| Polyvinyl alcohol | DIG Info | Debrisoquine 4-hydroxylase (EC50 = 354.8 uM) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Carmellose sodium | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [5] | |||
| Polyethylene glycol 3350 | DIG Info | Albendazole monooxygenase (Protein expression upregulation) | [5] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
