Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00421) | |||||
|---|---|---|---|---|---|
| Name |
Methyltestosterone
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
methyltestosterone; 17-Methyltestosterone; 58-18-4; Testred; Metandren; Android; 17alpha-Methyltestosterone; Androsan; Androsten; Mesterone; Malestrone; Mastestona; Synandrets; Synandrotabs; Testhormone; Andrometh; Anertan; Dumogran; Homandren; Hormale; Malogen; Masenone; Metestone; Metrone; Nabolin; Oraviron; Steronyl; Syndren; Testora; Oreton Methyl; Glosso-sterandryl; Oreton-M; M.T.Mucorettes; Neo-Hombreol-M; Nu-man; Android 5; Android 10; Android 25; Methitest; Orchisterone-M; Testovis Depot; Homandren, tablets; NSC-9701; Estratest; Metiltestosterona; Methyltestosteronum; 17beta-Hydroxy-17-methylandrost-4-en-3-one; 17-Methyltestosteron; CDB 110; UNII-V9EFU16ZIF; RU 24400; Anertan, tablets; 17-Hydroxy-17-methyl-3-keto-androstene-4; 17alpha-Methyl-3-oxo-4-androsten-17beta-ol; 17-beta-Hydroxy-17-methylandrost-4-en-3-one; 4-Androstene-17alpha-methyl-17beta-ol-3-one; A-Methyltestosterone; METHYL TESTOSTERONE; U 2842; V9EFU16ZIF; 17alpha-Methyl-delta-androsten-17beta-ol-3-one; L 589.372; CHEBI:27436; Oreton M; Androsan, tablets; component of Estan; (17beta)-17-hydroxy-17-methylandrost-4-en-3-one; Neo-Hombreol [M]; Neo-Homobreol (M); Neo-Homobreol [M]; component of Gynetone; NSC-139965; Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17b)-; NCGC00091009-03; component of Tylosterone; Glosso sterandryl; Testosterone, 17-methyl-; DSSTox_CID_13664; DSSTox_RID_79089; Anertan (tablets); DSSTox_GSID_33664; Homandren (VAN); Testovis (tablet); 17.alpha.-Methyltestosterone; 17.alpha.-Methyl-3-oxo-4-androsten-17.beta.-ol; Androsan (tablets); Androsan (VAN); Testoviron (VAN); Anertan (VAN); 4-Androstene-17.alpha.-methyl-17.beta.-ol-3-one; Androst-4-en-3-one, 17.beta.-hydroxy-17-methyl-; Testoviron (tablet); Oretron; Metiltestosterone; Estan (Salt/Mix); Metiltestosterone [DCIT]; Estratest HS; 17-alpha-Methyltestosterone; CAS-58-18-4; Estratest (Salt/Mix); Estratest H.S.; SMR000058528; Android (TN); CCRIS 3723; Methyltestosteronum [INN-Latin]; Metiltestosterona [INN-Spanish]; HSDB 3365; WLN: L E5 B666 OV MUTJ A E FQ F -B&AEF; EINECS 200-366-3; NSC 139965; BRN 2057425; component of Gynetone (Salt/Mix); Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17beta)-; Premarin with methyltestosterone; Estratest-HS; 4-Androstene-17-alpha-methyl-17-beta-ol-3-one; NCGC00091009-04; NCGC00091009-05; Androst-4-en-3-one, 17beta-hydroxy-17-methyl-; Testred (TN); (17-beta)-17-Hydroxy-17-methylandrost-4-en-3-one; Premarin with Methyltestosterone (Salt/Mix); 17(alpha)-Methyl-delta4-androsten-17(beta)-ol-3-one; 17-Hydroxy-17-methylandrost-4-en-3-one #; Methyltestosterone ciii; 17a-methyltestosterone; Methyltestosterone [USP:INN:BAN:JAN]; Conjugated estrogens / methyltestosterone; 17-.beta.-Hydroxy-17-methylandrost-4-en-3-one; Androst-4-en-3-on-17.beta.-ol, 17.alpha.-methyl; Androst-4-ene-17.alpha.-methyl-17.beta.-ol-3-one; 17.beta.-Hydroxy-17.alpha.-methylandrost-4-en-3-one; 17.alpha.-methyl-.DELTA.4-androsten-17.beta.-ol-3-one; CHEMBL1395; SCHEMBL18657; 4-08-00-01010 (Beilstein Handbook Reference); Androst-4-en-3-one, 17-beta-hydroxy-17-methyl-; Conjugated estrogens mixture with methyltestosterone; Methyltestosterone mixture with conjugated estrogens; MLS000759474; MLS001424040; MLS002174282; GTPL6945; DTXSID1033664; CDB-110; NSC9701; HMS2051A14; HMS2272A06; HY-A0121; ZINC3814422; Tox21_113161; Tox21_113162; Tox21_400058; BDBM50410531; LMST02020029; Methyltestosterone (JP17/USP/INN); NSC139965; AKOS015917317; Tox21_113162_1; ACN-031982; CCG-100871; CS-5099; DB06710; GS-6594; MCULE-5274452426; NC00121; NCGC00091009-01; NCGC00091009-06; NCGC00091009-07; Oxandrolone impurity, methyltestosterone-; 262423-02-9; RU-24400; SMR001261452; 17alpha-Methyltestosterone, >=97.0% (HPLC); C07198; D00408; Methyltestosterone (17alpha-Methyltestosterone); U-2842; AB00443683-06; AB00443683-09; 17alpha-methyl-Delta4-androsten-17beta-ol-3-one; 17alpha-Methyltestosterone, solid (photosensitive); 17beta-Hydroxy-17alpha-methyl-4-androsten-3-one; Q421768; 17alpha-methyl-Delta(4)-androsten-17beta-ol-3-one; L-589372; L-589.372; 17(alpha)-methyl-Delta(4)-androsten-17(beta)-ol-3-one; 17-alpha-Methyltestosterone 100 microg/mL in Acetonitrile; 17alpha-Methyltestosterone, VETRANAL(TM), analytical standard; Methyltestosterone, European Pharmacopoeia (EP) Reference Standard; Methyltestosterone (17alpha-Methyltestosterone) 1.0 mg/ml in Acetonitrile; Methyltestosterone, United States Pharmacopeia (USP) Reference Standard
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Breast cancer | ICD-11: 2C60 | [1] | ||
| PubChem CID | |||||
| Formula |
C20H30O2
|
||||
| Canonical SMILES |
C[C@]12CCC(=O)C=C1CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CC[C@]4(C)O)C
|
||||
| InChI |
1S/C20H30O2/c1-18-9-6-14(21)12-13(18)4-5-15-16(18)7-10-19(2)17(15)8-11-20(19,3)22/h12,15-17,22H,4-11H2,1-3H3/t15-,16+,17+,18+,19+,20+/m1/s1
|
||||
| InChIKey |
GCKMFJBGXUYNAG-HLXURNFRSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=6010"></iframe>
|
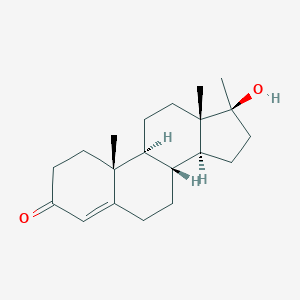 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 302.5 | Topological Polar Surface Area | 37.3 | |
| XlogP | 3.4 | Complexity | 550 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 0 | ||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Methyltestosterone 10 mg capsule | Click to Show/Hide the Full List of Formulation(s): 2 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
D&c yellow no. 10; Fd&c red no. 40; Fd&c blue no. 1; Fd&c blue no. 2; Magnesium stearate; Ferrosoferric oxide; Propylene glycol; Gelatin; Shellac; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Impax Generics | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [3] | |||
| FD&C blue no. 2 | DIG Info | Adenosine receptor A3 (IC50 = 1 uM) | [2] | |||
| Quinoline yellow WS | DIG Info | Estrogen receptor alpha (IC50 = 18 uM) | [2] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [4] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
| Drug Formulation 2 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Fd&c red no. 40; Fd&c blue no. 1; Gelatin, unspecified; Starch, corn
|
|||||
| Dosage Form | Oral Capsule | |||||
| Company | Valeant Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 4.7 uM) | [2] | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [3] | |||
| Allura red AC dye | DIG Info | Solute carrier SLCO2B1 (Ki = 2.59 uM) | [3] | |||
| Methyltestosterone 10 mg tablet | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Lactose monohydrate; Sodium lauryl sulfate; Magnesium stearate; Sucrose; Acacia; Guar gum; Powdered cellulose; Starch, corn
|
|||||
| Dosage Form | Oral Tablet | |||||
| Company | Impax Generics | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| Sodium lauryl sulfate | DIG Info | Solute carrier SLCO2B1 (Ki = 1.98 uM) | [3] | |||
| Magnesium stearate | DIG Info | Albendazole monooxygenase (Protein expression downregulation) | [5] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
