| Synonyms |
Click to Show/Hide the Synonyms of This API
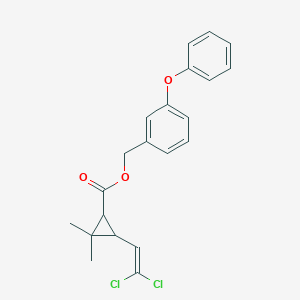
Permethrin; 52645-53-1; Ambush; Transpermethrin; Pounce; Elimite; Imperator; UNII-509F88P9SZ; NRDC-143; (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate; (+)-trans-Permethrin; Permethrine; Permetrina; Acticin; Ambushfog; Corsair; Dragnet; Ectiban; Kaleait; Kestrel; Outflank; Perigen; Permasect; Perthrine; Stomoxin; Stomozan; Coopex; Eksmin; Picket; Expar; Kafil; Kavil; Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester; Anomethrin N; Ridect pour-on; CHEBI:34911; 509F88P9SZ; 1RS cis-Permethrin; 52341-32-9; 1RS,cis-Permethrin; NCGC00159390-02; 1RS-trans-Permethrin; Kudos; Transpermethrin [ISO]; (+-)-cis-Permethrin; DSSTox_CID_2292; 3-phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; trans-(+-)-Permethrin; DSSTox_RID_76537; DSSTox_GSID_22292; 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropane carboxylic acid, (3-phenoxyphenyl) methyl ester; Permethrinum; S-3151; Chinetrin; Ecsumin; Efmethrin; Indothrin; Lyclear; NRDC 146; NRDC 148; Quamlin; Stomoxi; Cosair; Exmin; Exsmin; Ipitox; SBP-1513; (+-)-trans-Permethrin; trans-Permethrin D6 (dimethyl D6); Permethrine,c&t; (+-)-cis-Fmc 33297; Diffusil H; Insorbcid MP; Stomoxin P; Outflank-stockade; Perigen W; Dragnet FT; Picket G; Permethrin,racemic; [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; Mitin BC; Permanone 80; Permasect-25EC; FMC 35171; SMR000778043; Kestrel (pesticide); LE 79-519; Antiborer 3768; CAS-52645-53-1; Bematin 987; NRDC 143; Permethrinum [Latin]; Permetrin (Hungarian); Permitrene (Hungarian); Permetrina [Portuguese]; Caswell No. 652BB; FMC 33297; NIA 33297; PP 557; Permethrine [ISO-French]; BRN 4153590; Permethrn; Permethrin (isomers) solution; SBP-1513TEC; Permethrin [USAN:INN:BAN]; AI3-29296; CCRIS 2001; ICI-PP 557; MP79; SBP 15131TEC; 3-Phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate; HSDB 6790; m-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; BW-21-Z; S 3151; 82523-59-9; Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R-trans)-; OMS 1821; EINECS 258-067-9; Elimite (TN); FMC 41655; EPA Pesticide Chemical Code 109701; JF 7065; BRN 2063148; WL 43479; AI3-29158; Permethrin (USAN/INN); Hemoglobin atlanta-coventry; CHEMBL1525; SCHEMBL26543; Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, cis-(+-)-; Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, trans-(+-)-; MLS001332525; MLS001332526; Permethrin [ANSI:BSI:ISO]; Permethrin cis/trans ~ 1:1; Permethrin, analytical standard; DTXSID8022292; SCHEMBL15218274; HMS2232L22; HMS3264N07; HMS3369D10; Pharmakon1600-01504932; Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R,3S)-rel-; Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R-cis)-; HY-B0887; Tox21_111627; Tox21_201586; Tox21_300691; BBL005484; MFCD00041809; NSC760105; s6461; STL135986; AKOS005746953; CCG-213703; DB04930; KS-5079; MCULE-1256227237; NSC-760105; (+-)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; (3-Phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate; 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester; 3-Phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; 3-Phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; 3-Phenoxybenzyl(+-)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylate; m-Phenoxybenzyl (+-)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; m-Phenoxybenzyl (+1)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; Permethrin 10 microg/mL in Cyclohexane; NCGC00159390-00; NCGC00159390-04; NCGC00159390-05; NCGC00159390-06; NCGC00159390-07; NCGC00159390-08; NCGC00159390-09; NCGC00159390-10; NCGC00159390-11; NCGC00159390-12; NCGC00159390-13; NCGC00159390-14; NCGC00254599-01; NCGC00259135-01; Permethrin 100 microg/mL in Cyclohexane; (3-Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; 3-(Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; 52341-33-0; Permethrin (isomers), analytical standard; DB-052153; Total Permethrin 100 microg/mL in Acetone; 541-EP2274983A1; 541-EP2275422A1; 541-EP2280009A1; 541-EP2292608A1; 541-EP2298076A1; 541-EP2298077A1; 541-EP2301353A1; 541-EP2305031A1; 541-EP2305034A1; 541-EP2305035A1; 541-EP2305658A1; 541-EP2305662A1; 541-EP2308857A1; 541-EP2308858A1; 541-EP2311816A1; 541-EP2311817A1; 541-EP2314583A1; FT-0630656; ST50975270; Permethrin, PESTANAL(R), analytical standard; C14388; D05443; AB00918441_05; 645P531; Q411635; J-523915; Permethrin (25:75), EuropePharmacopoeia (EP) Reference Standard; (1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propanecarboxylate; 3-phenoxybenzyl 2-(2,2-dichlorovinyl)3,3-dimethylcyclopropanecarboxylate; 3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; (3-phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropanecarboxylate; (3-phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxyl ate; 3-phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate; m-phenoxybenzyl 2,2-dimethyl-3-(2',2'-dichlorovinyl)-cyclopropanecarboxylate; Permethrin for system suitability, EuropePharmacopoeia (EP) Reference Standard; (3-Phenoxyphenyl)methyl (+-)cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; (3-Phenoxyphenyl)methyl (+/-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; 93389-07-2; Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, 3-phenoxybenzyl ester, (+-)-, (cis,trans)-; Permethrin (isomers) solution, 100 mug/mL in acetonitrile, PESTANAL(R), analytical standard; Permethrin (isomers) solution, cis/trans isomers, 1000 mug/mL in methanol, analytical standard
|
 click to show the detail info of this DFM
click to show the detail info of this DFM
 click to show the detail info of this DFM
click to show the detail info of this DFM