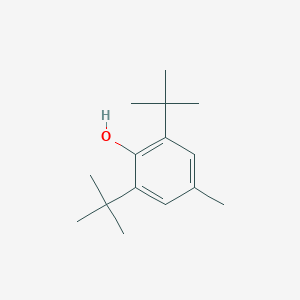
Details of the Drug Inactive Ingredient (DIG)
| Full List of Active Pharmaceutical Ingredients (APIs) Co-administrated with This DIG | ||||||
|---|---|---|---|---|---|---|
| ICD Disease Classification 01 | Infectious/parasitic disease | Click to Show/Hide | ||||
|
Azithromycin
|
API Info |
Congenital syphilis [ICD-11: 1A60]
|
[1] | |||
|
Raltegravir
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[2] | |||
|
Ritonavir
|
API Info |
Human immunodeficiency virus infection [ICD-11: 1C60]
|
[3] | |||
|
Fidaxomicin
|
API Info |
Clostridium difficile infection [ICD-11: 1A04]
|
[4] | |||
| ICD Disease Classification 02 | Benign/in-situ/malignant neoplasm | Click to Show/Hide | ||||
|
Prednisolone
|
API Info |
Multiple myeloma [ICD-11: 2A83]
|
[5] | |||
|
Everolimus
|
API Info |
Renal cell carcinoma [ICD-11: 2C90]
|
[6] | |||
|
Exemestane
|
API Info |
Breast cancer [ICD-11: 2C60]
|
[7] | |||
| ICD Disease Classification 04 | Immune system disease | Click to Show/Hide | ||||
|
Cetirizine
|
API Info |
Allergy [ICD-11: 4A80]
|
[8] | |||
| ICD Disease Classification 05 | Endocrine/nutritional/metabolic disease | Click to Show/Hide | ||||
|
Glipizide
|
API Info |
Diabetes mellitus [ICD-11: 5A10]
|
[9] | |||
|
Rosuvastatin
|
API Info |
Hypertriglyceridaemia [ICD-11: 5C80]
|
[10] | |||
|
Paricalcitol
|
API Info |
Hyperparathyroidism [ICD-11: 5A51]
|
[11] | |||
|
Calcifediol
|
API Info |
Vitamin deficiency [ICD-11: 5B55]
|
[12] | |||
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | ||||
|
Sertraline
|
API Info |
Major depressive disorder [ICD-11: 6A70]
|
[13] | |||
|
Methylphenidate
|
API Info |
Attention deficit hyperactivity disorder [ICD-11: 6A05]
|
[14] | |||
|
Escitalopram
|
API Info |
Major depressive disorder [ICD-11: 6A70]
|
[15] | |||
|
Paliperidone
|
API Info |
Bipolar disorder [ICD-11: 6A60]
|
[16] | |||
|
Desmopressin acetate
|
API Info |
Enuresis [ICD-11: 6C00]
|
[17] | |||
| ICD Disease Classification 08 | Nervous system disease | Click to Show/Hide | ||||
|
Morphine
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[18] | |||
|
Oxycodone
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[19] | |||
|
Hydromorphone
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[20] | |||
|
Hydrocodone
|
API Info |
Anaesthesia [ICD-11: 8E22]
|
[21] | |||
|
Deutetrabenazine
|
API Info |
Huntington disease [ICD-11: 8A01]
|
[22] | |||
| ICD Disease Classification 11 | Circulatory system disease | Click to Show/Hide | ||||
|
Aspirin
|
API Info |
Myocardial infarction [ICD-11: BA41]
|
[23] | |||
|
Verapamil
|
API Info |
Essential hypertension [ICD-11: BA00]
|
[24] | |||
|
Felodipine
|
API Info |
Essential hypertension [ICD-11: BA00]
|
[25] | |||
|
Amlodipine
|
API Info |
Angina pectoris [ICD-11: BA40]
|
[26] | |||
| ICD Disease Classification 13 | Digestive system disease | Click to Show/Hide | ||||
|
Metoclopramide
|
API Info |
Gastro-oesophageal reflux disease [ICD-11: DA22]
|
[27] | |||
|
Tacrolimus
|
API Info |
Ulcerative colitis [ICD-11: DD71]
|
[28] | |||
| ICD Disease Classification 16 | Genitourinary system disease | Click to Show/Hide | ||||
|
Oxybutynin
|
API Info |
Overactive bladder [ICD-11: GC50]
|
[29] | |||
|
Dutasteride
|
API Info |
Prostatic hyperplasia [ICD-11: GA90]
|
[30] | |||
| ICD Disease Classification 20 | Developmental anomaly | Click to Show/Hide | ||||
|
Calcitriol
|
API Info |
Congenital alopecia [ICD-11: LC30]
|
[31] | |||
| Full List of Biological Targets of DIG (DBTs) Regulated by This DIG | ||||||
|---|---|---|---|---|---|---|
| Lyase/isomerase/ligase (L/I/G) | ||||||
| DBT Name: Carbonic anhydrase I (CA1) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 245200 nM (tested by experiment) | [32] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CAH1_HUMAN | |||||
| DBT Name: Carbonic anhydrase II (CA2) | Click to Show/Hide | |||||
| Detailed Information |
DBT Info
 click to show the detail info of this DBT click to show the detail info of this DBT
|
|||||
| Experiment for Assessing the Biological Activity of the Studied DIG on This DBT | ||||||
| Biological Activity | Ki = 630 nM (tested by experiment) | [32] | ||||
| Tested Species | Homo sapiens (Human) | |||||
| UniProt ID | CAH2_HUMAN | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.