Details of the Active Pharmaceutical Ingredient (API)
| General Information of API (ID: D00089) | |||||
|---|---|---|---|---|---|
| Name |
Calcifediol
|
||||
| Synonyms |
Click to Show/Hide the Synonyms of This API
Calcifediol; Calcidiol; 25-hydroxyvitamin D3; 25-Hydroxycholecalciferol; 19356-17-3; Hidroferol; Calcifediol anhydrous; Didrogyl; 25-Hydroxyvitamin D; Calderol; Calcifediolum; Rayaldee; Ro 8-8892; 5,6-cis-25-Hydroxyvitamin D3; Cholecalciferol, 25-hydroxy-; UNII-T0WXW8F54E; CHEBI:17933; (3S,5Z,7E)-9,10-secocholesta-5,7,10-triene-3,25-diol; T0WXW8F54E; MFCD00867077; NCGC00161326-04; Calcifidiol; Delakmin; (3beta,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-3,25-diol; 63283-36-3; (5Z,7E)-(3S)-9,10-secocholesta-5,7,10(19)-triene-3,25-diol; 25-Hydroxycholecalciferol monohydrate; U-32070E; U 32070 E; 25-hydroxyvitamin D3 / 25-hydroxycholecalciferol / calcidiol; 25-Hydroxycholescalciferol; Calcifediolum [INN-Latin]; Rovimix Hy-D; Calcifediol [INN]; (3b,5Z,7E)- 9,10-Secocholesta-5,7,10(19)-triene-3,25-diol; 36149-00-5; 25-(OH)Vitamin D3; 25-Hydroxyvitamin D3 solution; 5,6-trans-25-Hydroxycholescalciferol; Ryaldee; BML2-E02; (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatriene-3,25-diol; 25(OH)D3; EINECS 242-990-9; 78782-98-6; PubChem18824; (5Z,7E)-9,10-Seco-5,7,10(19)-cholestatrien-3beta,25-diol; 5,6-trans-9,10-Seco-5,7,10(19)-cholestatrien-3beta,25-diol; Spectrum5_001931; DSSTox_CID_2721; 25-hydroxy-cholecalciferol; Vitamin D, 25-hydroxy-; SCHEMBL3296; CHEMBL1040; DSSTox_RID_76699; DSSTox_GSID_22721; BSPBio_001411; (3S,5Z,7E)-9,10-seco-5,7,10(19)-cholestatriene-3,25-diol; GTPL6921; DTXSID0022721; CHEBI:94743; BCPP000306; HMS1361G13; HMS1791G13; HMS1989G13; HMS2089L21; HMS3402G13; ACT06833; ZINC4474414; Tox21_111987; (3S,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-3,25-diol; 9,10-Secocholesta-5,7,10(19)-triene-3,25-diol, (3beta,5Z,7E)-; BDBM50521013; LMST03020246; AKOS015965097; BCP9000472; CCG-268657; CS-0800; DB00146; IDI1_033881; NCGC00161326-01; AC-31367; HY-32351; CAS-19356-17-3; 25-Hydroxycholecalciferol, >=98% (HPLC); C01561; W-5159; AB01275461-01; AB01275461_02; 356C173; Q139307; SR-05000001468; SR-05000001468-1; W-201718; 25-Hydroxyvitamin D3 monohydrate, >=99.0% (HPLC); 9,10-Secocholesta-5,7,10(19)-triene-3b,25-diol; BRD-K77175907-001-01-5; 25-Hydroxyvitamin D3-23,24,25,26,27-13C5 solution; B91135EC-8937-4D8B-A533-CCD82F33C1B0; Calcifediol, European Pharmacopoeia (EP) Reference Standard; (5E,7E)-9,10-Secocholesta-5,7,10(19)-triene-3beta,25-diol; 25-Hydroxyvitamin D3 solution, 100 mug/mL in ethanol, 98% (CP); 25-Hydroxyvitamin D3 solution, 5 mug/mL in ethanol, 98% (CP); 25-Hydroxyvitamin D3 solution, 50 mug/mL in ethanol, 98% (CP); Calcifediol, United States Pharmacopeia (USP) Reference Standard; (3S,5Z,14beta,17alpha)-9,10-secocholesta-5,7,10-triene-3,25-diol; 9,10-Secocholesta-5,7,10(19)-triene-1,25-diol, (3.beta,.5Z,7E)-; 64719-49-9
|
||||
| Clinical Status |
Approved
|
||||
| Disease Indication | Vitamin deficiency | ICD-11: 5B55 | [1] | ||
| PubChem CID | |||||
| Formula |
C27H44O2
|
||||
| Canonical SMILES |
C[C@H](CCCC(C)(C)O)[C@H]1CC[C@@H]\\2[C@@]1(CCC/C2=C\\C=C/3\\C[C@H](CCC3=C)O)C
|
||||
| InChI |
1S/C27H44O2/c1-19-10-13-23(28)18-22(19)12-11-21-9-7-17-27(5)24(14-15-25(21)27)20(2)8-6-16-26(3,4)29/h11-12,20,23-25,28-29H,1,6-10,13-18H2,2-5H3/b21-11+,22-12-/t20-,23+,24-,25+,27-/m1/s1
|
||||
| InChIKey |
JWUBBDSIWDLEOM-DTOXIADCSA-N
|
||||
| Click to Show/Hide the Molecular Data (Structure/Property) of This API | |||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5283731"></iframe>
|
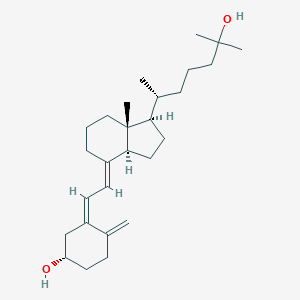 |
|||
| 3D MOL | 2D MOL | ||||
| Physicochemical Properties | Molecular Weight | 400.6 | Topological Polar Surface Area | 40.5 | |
| XlogP | 6.2 | Complexity | 655 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 6 | ||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | ||
| Full List of Drug Formulations (DFMs) Containing This API | ||||||
|---|---|---|---|---|---|---|
| Calcifediol 0.03 mg capsule | Click to Show/Hide the Full List of Formulation(s): 1 Formulation(s) | |||||
| Drug Formulation 1 |
DFM Info
 click to show the detail info of this DFM click to show the detail info of this DFM
|
|||||
| All DIGs | Click to Show/Hide the Full List of DIGs in This DFM
Butylated hydroxytoluene; Fd&c blue no. 1; Isopropyl alcohol; Sorbitol; Propylene glycol; Titanium dioxide; Water; Medium-chain triglycerides; Alcohol; Carrageenan; Diacetyltartaric and fatty acid esters of glycerol; Hydroxypropyl corn starch (5% substitution by weight); Hypromelloses; Lauroyl peg-32 glycerides; Mineral oil; Paraffin; Sodium phosphate, dibasic; Sorbitan
|
|||||
| Dosage Form | 24 HR Extended Release Oral Capsule | |||||
| Company | OPKO Pharmaceuticals | |||||
| DIG(s) with Biological Activity | ||||||
| DIG Name | DIG Info | Representative Biological Activity of This DIG | REF | |||
| FD&C blue no. 1 | DIG Info | Solute carrier SLCO2B1 (Ki = 13 uM) | [2] | |||
| Butylated hydroxytoluene | DIG Info | Carbonic anhydrase II (Ki = 630 nM) | [3] | |||
| Isopropyl alcohol | DIG Info | Lymphoma P388/ADR cells (IC50 = 0.22 uM) | [4] | |||
| Propylene glycol | DIG Info | Albendazole monooxygenase (Inhibition ratio < 20 %) | [5] | |||
| Medium-chain triglyceride | DIG Info | Colon cancer Caco-2 cells (Inhibition ratio > 36 %) | [6] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Zhang and Dr. Mou.
